Abstract
The capnodynamic method is a minimally invasive method continuously calculating effective pulmonary blood flow (COEPBF), equivalent to cardiac output when intra pulmonary shunt flow is low. The capnodynamic equation joined with a ventilator pattern containing cyclic reoccurring expiratory holds, provides breath to breath hemodynamic monitoring in the anesthetized patient. Its performance however, might be affected by changes in the mixed venous content of carbon dioxide (CvCO2). The aim of the current study was to evaluate COEPBF during rapid measurable changes in mixed venous carbon dioxide partial pressure (PvCO2) following ischemia–reperfusion and during sustained hypercapnia in a porcine model. Sixteen pigs were submitted to either ischemia–reperfusion (n = 8) after the release of an aortic balloon inflated during 30 min or to prolonged hypercapnia (n = 8) induced by adding an instrumental dead space. Reference cardiac output (CO) was measured by an ultrasonic flow probe placed around the pulmonary artery trunk (COTS). Hemodynamic measurements were obtained at baseline, end of ischemia and during the first 5 min of reperfusion as well as during prolonged hypercapnia at high and low CO states. Ischemia–reperfusion resulted in large changes in PvCO2, hemodynamics and lactate. Bias (limits of agreement) was 0.7 (−0.4 to 1.8) L/min with a mean error of 28% at baseline. COEPBF was impaired during reperfusion but agreement was restored within 5 min. During prolonged hypercapnia, agreement remained good during changes in CO. The mean polar angle was −4.19° (−8.8° to 0.42°). Capnodynamic COEPBF is affected but recovers rapidly after transient large changes in PvCO2 and preserves good agreement and trending ability during states of prolonged hypercapnia at different levels of CO.
Similar content being viewed by others
1 Introduction
Hemodynamic optimisation in high-risk surgery patients using goal directed protocols has shown to improve postoperative outcomes and is assumed to be cost effective [1,2,3,4,5]. A flow based parameter like cardiac output (CO) is ideally used to guide hemodynamic support [3]. Patients accepted for surgery nowadays tend to be older and carry more co-morbidity, thus expanding the high-risk group [6]. This underlines the need for a feasible CO monitor which is both fast and accurate during hemodynamic changes [7]. Several new methods for CO monitoring have emerged in recent years but have different limitations and most have not been adequately evaluated during hemodynamic alterations [8, 9].
Alveolar carbon dioxide concentration in expired gas can be easily measured in mechanically ventilated patients. By inducing measurable changes in alveolar carbon dioxide concentration, non-shunted pulmonary blood flow to the lungs can be calculated applying a differential Fick principle [10,11,12].
Our research group has previously described a capnodynamic method that continuously calculates effective pulmonary blood flow (COEPBF) based on small changes in CO2 concentration obtained from short expiratory pauses in three out of every nine breaths, conveniently automated by the ventilator [13,14,15]. COEPBF can be used as a surrogate for CO when intrapulmonary shunt is low and has shown fair agreement and good trending ability during significant hemodynamic and ventilatory alterations in experimental models [13,14,15,16].
Theoretically, the capnodynamic equation is vulnerable to changes in mixed-venous carbon dioxide concentration (CvCO2) as the equation system assumes it to remain constant during a measurement cycle. Clinical entities that might cause changes in CvCO2 such as reperfusion and hypercapnia are commonly seen in the perioperative period, for example in aortic and laparoscopic surgery. The aim of this study was to evaluate the performance of the capnodynamic method during ischemia–reperfusion and during prolonged hypercapnia induced by an increased dead space in a porcine model.
2 Methods
The study was approved by the Uppsala animal research ethical committee (nr. C 47/15, chairperson Kurt Ek) on April 24th 2015 and performed at the Hedenstierna laboratory in Uppsala University, Sweden.
Hällsjö Sander et al. have previously described the anaesthetic and surgical procedure in detail [13]. Briefly, 16 pigs with a mean weight of 36 kg (range 32–44 kg) were anaesthetised and mechanically ventilated in a volume-controlled mode with a tidal volume (TV) of 8 mL/kg, FIO2 0.40 and PEEP 5cmH2O (Servo-i, Maquet Critical Care, Solna, Sweden). Core temperature was maintained at 38–39 °C.
Eight animals underwent the ischemia/reperfusion protocol and eight animals the hypercapnia protocol. The animals used in this study were submitted to other separate experimental steps, not affecting the study protocol, that have been published in abstract form [17].
At the end of the protocol animals were sacrificed by a potassium chloride injection.
2.1 Instrumentation and equipment
A 20G catheter was placed in the carotid artery for arterial pressure monitoring and blood gas sampling. A 7.5Fr pulmonary artery catheter (Edwards Lifesciences Corp., Irvine, CA, USA) was inserted via the internal jugular vein for analysis of mixed venous blood gases, pulmonary arterial pressure and cardiac output (COPAC) via thermodilution. A 10Fr, 80 cm thrombectomy catheter (Dispomedica GmbH, Hamburg, Germany) was inserted under ultrasound guidance into the inferior cava vein via the femoral vein to allow controlled preload reduction with balloon inflation.
An ultrasonic flow probe was placed around the pulmonary trunk through a left-sided mini-thoracotomy for CO measurements (COTS) (T 401; Transonic Systems Inc., Ithaca NY, USA).
In the ischemia–reperfusion pigs a 12Fr stent graft balloon catheter (Reliant®, Medtronic Inc. Minneapolis, MN, USA) was inserted into the femoral artery with ultrasound guidance and placed in the abdominal aorta just beneath the diaphragm, verified with fluoroscopy.
Expired carbon dioxide was measured by a mainstream infrared sensor (Capnostat-3, Respironics Inc, Wallingford, CT, USA) and gas flow was analysed by the flow sensor incorporated in the ventilator which was connected to a computer where all the mathematical analysis was carried out with a software written in Matlab™ (The Mathworks Inc, Natick, MA, USA).
Blood gas analysis was performed by ABL-800FLEX (Radiometer Medical ApS, Brønshøj, Denmark).
Haemodynamic parameters were retrieved into a data acquisition system (Acknowledge, version 3.2.7, Bio Pac Systems, Santa Barbara, CA, USA).
2.2 Calculations and measurements of cardiac output, shunt and dead space
The capnodynamic method has previously been reported [15] and a detailed description can be found in the supplementary material. Briefly, a short pause is introduced to the expiratory phase of three out of nine breaths, automatically controlled by the ventilator. The resulting small differences (4–8 mmHg) in the alveolar concentration of CO2 between breaths can be inserted into the capnodynamic equation, describing the mole balance of CO2 transported to and from the lungs. Each breath creates one equation and with a stack of nine equations the COEPBF can be calculated using a least square-error optimization. With each breath the last equation is replaced with the newest allowing a continuous calculation of COEPBF, the cardiac output minus the intrapulmonary shunt, with each presented value representing an average of nine preceding breaths.
The reference method, COTS, represents the pulmonary blood flow (shunt included) generated by each cardiac cycle measured at the pulmonary trunk. Each measurement was based on an average of approximately 5–10 s.
COPAC, a well-known clinical method to measure CO (shunt included) was used as a reference in case of technical failure. Values were calculated averaging three intermittent thermodilutions always performed after the COTS and COEPBF measurements to avoid the short acting effect of cold saline on heart rate.
Shunt fraction was calculated using Berggren’s formula [18].
Enghoff’s dead space was measured using volumetric capnography (NICO monitor, Respironics, Wallingford, CT, USA) [19].
2.3 Experimental protocol
After instrumentation and subsequent 15 min stabilisation, precision and baseline (BL) measurements of COTS, COEPBF and COPAC were obtained at PEEP 5cmH2O and TV of 8 mL/kg.
2.4 Ischemia/reperfusion
Caudal ischemia was induced in eight pigs by inflation of the aortic balloon for approximately 30 min. The absence of blood flow was confirmed by ultrasound Doppler in the contralateral femoral artery. Severe increases in systemic vascular resistance (SVR) and mean arterial pressure (MAP) during the ischemic period were attenuated with high dose sodium nitroprusside infusion and intermittent beta-blocker boluses. In some animals the aortic balloon was temporarily released and then inflated again if the systolic blood pressure was >200 mmHg, despite maximal pharmacological treatment. Hemodynamic measurements and blood gases were obtained at baseline (BL) and at the end of ischemia, before balloon release. During the reperfusion phase COEPBF and COTS measurements were obtained simultaneously every minute and COPAC at minute 1, 3 and 5.
2.5 Hypercapnia
Prolonged hypercapnia was induced in eight animals by adding an external dead space at the airway opening between the y-piece and the carbon dioxide sensor, aiming for a 50–60% increase in PaCO2. Measurements were obtained at baseline, after establishing a stable hypercapnia on average 44 (8) min later and at 7–12 min intervals between baseline, cava balloon inflation, baseline and dobutamine infusion, aiming for ±30% change in CO. COEPBF and COTS readings were obtained simultaneously.
2.6 Statistics
D’Agostino and Pearson omnibus K2 test was used to check data for normal distribution. Results are presented as mean (standard deviation, SD). Proportional bias, i.e., the spread of bias at different CO levels, was checked with visual assessment and a linear regression. All statistical calculations except for the polar plots and confidence intervals (CI) were performed in Graph Pad Prism (version 6.0 for Windows, Graph Pad Software, La Jolla, CA, USA). For calculation of polar plots an excel sheet for conversion of Cartesian data to polar coordinates was used (kindly provided by Professor L Critchley) and displayed as graphs in Medcalc Statistical Software version 16.8.4 (MedCalc Software bvba, Ostend, Belgium) [20]. Calculations of CI were performed in excel (version 2007) with a t-table representing degrees of freedom, in accordance with the current discussion on comparison of different cardiac output monitors [21].
The animal was allowed to stabilise during and between each hemodynamic measurement during hypercapnia and therefore a correction for repeated measurements was not applied [21, 22].
2.7 Inherent precision
Inherent precision (defined as twice the coefficient of variation (CV = SDmethod/mean COmethod) of COEPBF was calculated from 10 measurements obtained at 1 min intervals in each animal at baseline conditions [23]. The previously reported precision for COTS was ±10% [24] and between ±8 and ±24% for COPAC depending on different haemodynamic conditions, ventilation, temperature and positioning of the catheter per se [25, 26]. Precision for COEPBF was ±14% during steady state conditions in our previous study [15].
2.8 Absolute values
Bland Altman methodology was used to measure the mean difference (bias) and the precision (levels of agreement) between COEPBF and COTS [27,28,29]. Proportional bias was checked with regression analysis to see if the slope deviated from zero. Due to small sample size the mean error (ME), also known as percentage error, used to estimate the precision of agreement was calculated as 100% × tα,n−1 × SDbias/mean COref, where tα,n−1 is the t-value corresponding to the degrees of freedom (n − 1) and a type I error (α) of 0.05 [21, 29].
Confidence intervals (CI) were calculated for all analyses, as described above. A priori, COEPBF was considered interchangeable to COTS if mean error was less than 30% [29], although less than 45% might be considered in settings of extensive hemodynamic changes or apparent advantage of the reference method [30].
2.9 Trending ability
The four-quadrant and the polar plot methodology were used to assess the agreement between test and reference methods regarding the direction and magnitude of the change [20]. Concordance rate for the four-quadrant plot was calculated as the number of data points in the two quadrants of agreement divided by the total number of data points, expressing the agreement between the paired delta-CO values for both methods [20]. Concordance rate for the polar plot was calculated as the number of data points within the radial limits of agreement of ±30° divided by the total number of data points [20].
Because of the high precision of the reference method, <20%, an exclusion zone of 10% was used [31]. We considered a concordance rate of >92 and >90% calculated by the four-quadrant plot and the polar plot methodology as good, respectively [32]. An angular bias smaller than ±5° indicated sufficient calibration between the test and the reference method [20, 32].
3 Results
All animals survived the experimental protocol that resulted in notable hemodynamic changes. The calculated inherent precision of COEPBF, COTS and COPAC during initial baseline conditions was 8, 4 and 10%, respectively. All data were normally distributed. Proportional bias between COEPBF and COTS was undetected in all interventions except during preload reduction in the hypercapnia protocol.
3.1 Ischemia/reperfusion
At the end of ischemia, a decrease in PvCO2 and increase in serum lactate were observed. Immediately after balloon release, PaCO2 and PvCO2 increased dramatically and remained high during the initial reperfusion phase (Table 1). Cardiac output varied throughout the protocol (Table 1) and other hemodynamic parameters were largely affected (Table 2). COEPBF and COTS showed bias (LoA) 0.7 (−0.4 to 1.8) L/min and ME 28% at baseline. The performance of COEPBF deteriorated immediately after balloon release but was restored gradually over the next 5 min (Fig. 1; Table 2).
Timeline showing a mean (SD) values for COEPBF, COTS and COPAC from baseline (BL) to end of ischemia (EOI) approximately 30 min later and at minute 1, 3 and 5 after reperfusion, and b continuous values from one animal for COEPBF and COTS (not possible with COPAC) from 2 min before balloon release (vertical broken line) and up to 8 min after reperfusion
3.2 Hypercapnia
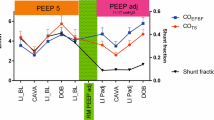
During prolonged hypercapnia, PaCO2 and PvCO2 increased from 5.6 (0.42) to 9.2 (0.5) kPa, and 7.41 (0.69) to 10.5 (0.91) kPa, respectively. See Table 2 for all hemodynamic, ventilatory and metabolic values.
At hypercapnia baseline conditions, COEPBF bias (LoA) was 0.5 (−0.5 to 1.4) L/min and ME was 21%.
The corresponding values at preload reduction and inotropic stimulation were 0.4 (−0.7 to 1.4) and 0.04 (−2.8 to 2.8) L/min, respectively. ME was 38% in both conditions. See Fig. 2 for visual assessment of the Bland Alman plot during hypercapnia and Table 1 for Bland Altman values for all interventions separately.
Bland–Altman plot showing 40 paired values for COEPBF vs COTS during dead space induced hypercapnia at baseline (blue dots), low CO (light blue triangles) and high CO (purple quadrants). Bias is represented with a whole blue line with corresponding CI (blue dotted lines) and levels of agreement (LoA) are shown with broken grey lines with corresponding CI (grey dotted lines)
3.3 Trending during hypercapnia
The concordance rate for COEPBF and COTS following preload reduction and inotropic stimulation was 100% according to both four quadrant and polar plot methodologies. The mean (95% CI) polar angle was −4.19° (−8.8° to 0.42°) (Fig. 3).
Polar plot for COEPBF with COTS as the reference method during dead space induced hypercapnia. The radial length is the mean of the pairwise delta values of the reference method and the test method (L/min) and is shown with a whole black line with corresponding CI (dotted lines). Data spread closely to the polar axis (orange dotted lines) indicate good trending
4 Discussion
We have evaluated the performance of a capnodynamic method to estimate COEPBF in a porcine model of ischemia/reperfusion and hypercapnia. As expected, agreement was impaired during reperfusion due to the sudden flux of carbon dioxide into the circulation. However, agreement was restored within 5 min. Prolonged hypercapnia did not impair agreement or trending ability of this method.
Anaesthetists working in the operating room are well aware that rapid physiological changes are to be expected. However, the performance of most non-invasive CO monitors has been found to be less reliable during rapid changes in vascular volume and resistance, a common feature during major surgery [8, 9].
The Fick’s principle, using elimination of CO2 from the lungs and the difference in concentration of CO2 in mixed venous and arterial blood is a well proven gold standard to calculate CO, although unsuitable for clinical use [33]. Modifications of Fick’s principle based on end tidal CO2 measurements, assuming a steady state in CVCO2, have been used to calculate effective pulmonary blood flow indirectly, which equals CO minus the shunt flow [34]. These indirect methods are dependent on induced alterations in alveolar CO2 accomplished by three means; (1) changes in the alveolar ventilation as originally described by Gideon et al. [10] and applied with modifications in our capnodynamic method, (2) changes in dead space as originally described by Capek et al. [35] and used in the NICO system with different software versions [36, 37] and (3) adding carbon dioxide to the inspiratory gas.
The capnodynamic method is continuous with short response time and needs no additional devices mounted to the y-piece, as opposed to the NICO monitor. Originally, inspiratory pauses where used to obtain the desired changes in alveolar ventilation. Good trending was observed during hemodynamic changes and lung injury but agreement (bias) and precision (LoA) was affected at higher PEEP and shunt levels in a porcine model [13, 14]. Following refinement changing to expiratory instead of inspiratory pauses, both agreement and precision improved when compared with the highly invasive ultrasonic flow probe and thermodilution based methods during significant hemodynamic and ventilatory changes [13,14,15,16].
A prerequisite for the capnodynamic equation is a stable concentration of CvCO2 during the measurement cycle. Therefore, we wanted to test the hypothesis that large alterations in venous concentration of carbon dioxide, as might be seen during major surgery, laparoscopy and lung protective ventilation would impair the performance of COEPBF. Rapid influx of carbon dioxide into the circulation is to be expected during vascular surgery following periods of ischemia, whereas in laparoscopic and robotic assisted surgery, insufflation of carbon dioxide induces prolonged hypercapnia. These clinical situations are common features in the operating theatre. The current protocol was designed to evaluate the performance of COEPBF in an animal model mimicking these clinical scenarios.
In the current study, ischemia and subsequent reperfusion were accomplished by aortic occlusion simulating suprarenal clamping during aortic surgery. The balloon inflation created severe hemodynamic changes in the animal, including a sudden massive increase in systemic vascular resistance demanding treatment with vasodilatory and rate regulatory drugs. As a result, pulmonary shunt increased from 9 to 26% at the end of ischemia and although decreasing during reperfusion, shunt remained elevated from baseline. Since COEPBF does not detect shunt flow, this contributed to the underestimation of CO at the end of ischemia (see Fig. 1; Table 1). As the capnodynamic equation requires a stable concentration of venous carbon dioxide during the measurement cycle in order to be accurate, COEPBF increased erroneously following the aortic balloon release due to the resulting dramatic rise in alveolar concentration of carbon dioxide. Despite the massive reperfusion, the performance of COEPBF was nearly fully restored within 5 min.
Prolonged hypercapnia was induced by adding dead space to the breathing circuit to maintain similar tidal volumes and respiratory rate and to mimic the hypercapnia often seen in laparoscopic surgery. Cardiac output, dead space, shunt, PaCO2 and PvCO2 increased as expected but did not affect bias, LoA or ME (see Table 2). These results indicate that the concentration of carbon dioxide per se does not impair the performance of the method in contrast to sudden changes. The performance of many CO monitoring techniques is impaired during rapid hemodynamic changes and requires frequent calibration in order to maintain accuracy. The capnodynamic method recuperated within 5 min after reperfusion. This time span is comparable to the duration of the calibration procedures of the thermodilution based CO monitors [30, 38].
The capnodynamic method also includes an internal control function that detects major incongruities that do not fit with the ideal capnodynamic equation. The inherent control function, not used in this study, has the potential to filter out unstable values as those seen in the reperfusion phase, supporting the clinician with only stable calculations of pulmonary blood flow.
In the statistical analysis we adapted to the most recent guidelines on comparing different cardiac output monitors [21]. Taken our previous studies and animal-research ethics into account we used as few animals as possible, despite an expected effect on confidence intervals and higher risk for proportional bias. The physiological stresses imposed on the animals in this study were extreme and the results needs to be interpreted in this context. At baseline the capnodynamic method showed acceptable bias, LoA and ME. Five minutes after massive reperfusion, notable increase in CO and large reduction in SVR the bias was almost zero but LoA and ME had increased. We believe this is acceptable for a continuous minimally invasive method complimentary to mechanical ventilation in anesthetized subjects, as long as trending is preserved, as previously suggested by Peyton and Chong [30].
This study has several limitations. As the capnodynamic method measures carbon dioxide in expired gas, the mixed-venous carbon dioxide bypassing the lungs i.e., shunted, is not detected. In the current study, we have utilized CO as a reference for comparison. Since the reference method and the test method do not measure identical physiological variables, a difference is to be expected. Our previous studies have shown that COEPBF has acceptable agreement when shunt is below 20%. At higher shunt fractions the agreement is impaired, however, the trending ability is still reliable [13,14,15].
The major hemodynamic changes in the protocol required intermittent intense pharmacologic support. This resulted in inter-individual differences in treatment and physiological status between animals and thus, a less homogenous model.
It could also be reasoned that with the magnitude of these circulatory changes, the trending ability is anticipated to be aligned. However, we believe that it is of utmost importance to challenge new monitoring technologies in extreme conditions which is ethically not possible to induce in humans.
In conclusion COEPBF maintained a good agreement during hypercapnia and was marginally affected during severe ischemia. Only 5 min after massive reperfusion agreement returned to previous acceptable levels. Clinical studies on COEPBF are underway.
References
Marik PE. Perioperative hemodynamic optimization: a revised approach. J Clin Anesth. 2014;26(6):500–5. doi:10.1016/j.jclinane.2014.06.008.
Waldron NH, Miller TE, Gan TJ. Perioperative goal-directed therapy. J Cardiothorac Vasc Anesth. 2014;28(6):1635–41. doi:10.1053/j.jvca.2014.07.008.
Gurgel ST, do Nascimento Jr P. Maintaining tissue perfusion in high-risk surgical patients: a systematic review of randomized clinical trials. Anesth Analg. 2011;112(6):1384–91. doi:10.1213/ANE.0b013e3182055384.
Ebm C, Cecconi M, Sutton L, Rhodes A. A cost-effectiveness analysis of postoperative goal-directed therapy for high-risk surgical patients. Crit Care Med. 2014;42(5):1194–203. doi:10.1097/CCM.0000000000000164.
Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103(5):637–46. doi:10.1093/bja/aep279.
Huisman MG, Kok M, de Bock GH, van Leeuwen BL. Delivering tailored surgery to older cancer patients: preoperative geriatric assessment domains and screening tools—a systematic review of systematic reviews. Eur J Surg Oncol. 2016. doi:10.1016/j.ejso.2016.06.003.
Moonesinghe SR, Mythen MG, Grocott MP. High-risk surgery: epidemiology and outcomes. Anesth Analg. 2011;112(4):891–901. doi:10.1213/ANE.0b013e3181e1655b.
Thiele RH, Bartels K, Gan TJ. Cardiac output monitoring: a contemporary assessment and review. Crit Care Med. 2015;43(1):177–85. doi:10.1097/CCM.0000000000000608.
Thiele RH, Bartels K, Gan TJ. Inter-device differences in monitoring for goal-directed fluid therapy. Can J Anaesth. 2015;62(2):169–81. doi:10.1007/s12630-014-0265-z.
Gedeon A, Forslund L, Hedenstierna G, Romano E. A new method for noninvasive bedside determination of pulmonary blood flow. Med Biol Eng Comput. 1980;18(4):411–8.
Gedeon A, Krill P, Osterlund B. Pulmonary blood flow (cardiac output) and the effective lung volume determined from a short breath hold using the differential Fick method. J Clin Monit Comput. 2002;17(5):313–21. doi:10.1023/A:1021232624767.
Peyton PJ, Thompson D, Junor P. Non-invasive automated measurement of cardiac output during stable cardiac surgery using a fully integrated differential CO(2) Fick method. J Clin Monit Comput. 2008;22(4):285–92. doi:10.1007/s10877-008-9131-2.
Hällsjö Sander C, Hallback M, Wallin M, Emtell P, Oldner A, Bjorne H. Novel continuous capnodynamic method for cardiac output assessment during mechanical ventilation. Br J Anaesth. 2014;112(5):824–31. doi:10.1093/bja/aet486.
Hällsjö Sander C, Hallback M, Suarez Sipmann F, Wallin M, Oldner A, Bjorne H. A novel continuous capnodynamic method for cardiac output assessment in a porcine model of lung lavage. Acta Anaesthesiol Scand. 2015. doi:10.1111/aas.12559.
Sander CH, Sigmundsson T, Hallback M, Sipmann FS, Wallin M, Oldner A, Bjorne H. A modified breathing pattern improves the performance of a continuous capnodynamic method for estimation of effective pulmonary blood flow. J Clin Monit Comput. 2016. doi:10.1007/s10877-016-9891-z.
Peyton P. Minimally invasive monitoring of cardiac output and lung gas exchange: taking it mainstream. J Clin Monit Comput. 2016. doi:10.1007/s10877-016-9866-0.
Sigmundsson T, Öhman T, Redondo E, Hallbäck M, Wallin M, Suarez Sipmann F, Oldner A, Hällsjö Sander C, Björne H. A capnodynamic method for monitoring effective pulmonary blood flow—evaluation during hypercapnia. Intensive Care Med Exp. 2016;4(Suppl 1):A315.
Berggren S. The oxygen deficit of arterial blood caused by non-ventilation parts of the lung. Acta Physiol Scand. 1942;4(Suppl 2):1–92.
Tusman G, Sipmann FS, Bohm SH. Rationale of dead space measurement by volumetric capnography. Anesth Analg. 2012;114(4):866–74. doi:10.1213/ANE.0b013e318247f6cc.
Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth. 2011;25(3):536–46. doi:10.1053/j.jvca.2011.01.003.
Montenij LJ, Buhre WF, Jansen JR, Kruitwagen CL, de Waal EE. Methodology of method comparison studies evaluating the validity of cardiac output monitors: a stepwise approach and checklist. Br J Anaesth. 2016;116(6):750–8. doi:10.1093/bja/aew094.
Hapfelmeier A, Cecconi M, Saugel B. Cardiac output method comparison studies: the relation of the precision of agreement and the precision of method. J Clin Monit Comput. 2015. doi:10.1007/s10877-015-9711-x.
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds RM. Bench-to-bedside review: the importance of the precision of the reference technique in method comparison studies—with specific reference to the measurement of cardiac output. Crit Care. 2009;13(1):201. doi:10.1186/cc7129.
Transonic Systems Inc. Transonic precision flowprobes: perivascular flowprobe specifications. Technical Report (2011).
Yang XX, Critchley LA, Rowlands DK, Fang Z, Huang L. Systematic error of cardiac output measured by bolus thermodilution with a pulmonary artery catheter compared with that measured by an aortic flow probe in a pig model. J Cardiothorac Vasc Anesth. 2013;27(6):1133–9. doi:10.1053/j.jvca.2013.05.020.
Kirkeby-Garstad I, Tronnes H, Stenseth R, Sellevold OF, Aadahl P, Skogvoll E. The precision of pulmonary artery catheter bolus thermodilution cardiac output measurements varies with the clinical situation. J Cardiothorac Vasc Anesth. 2015;29(4):881–8. doi:10.1053/j.jvca.2014.12.016.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82. doi:10.1080/10543400701329422.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15(2):85–91.
Peyton PJ, Chong SW. Minimally invasive measurement of cardiac output during surgery and critical care: a meta-analysis of accuracy and precision. Anesthesiology. 2010;113(5):1220–35. doi:10.1097/ALN.0b013e3181ee3130.
Axiak Flammer SM, Critchley LA, Weber A, Pirbodaghi T, Brinks H, Vandenberghe S. Reliability of lithium dilution cardiac output in anaesthetized sheep. Br J Anaesth. 2013;111(5):833–9. doi:10.1093/bja/aet220.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111(5):1180–92. doi:10.1213/ANE.0b013e3181f08a5b.
Löwy A, v Schrötter H. Untersuchungen uber die Blutcirkulation beim Menschen. Zeitschr f exp Pathol u Therapie. 1905;1:197–311.
Weinbroum AA, Biderman P, Soffer D, Klausner JM, Szold O. Reliability of cardiac output calculation by the fick principle and central venous oxygen saturation in emergency conditions. J Clin Monit Comput. 2008;22(5):361–6. doi:10.1007/s10877-008-9143-y.
Capek JM, Roy RJ. Noninvasive measurement of cardiac output using partial CO2 rebreathing. IEEE Trans Biomed Eng. 1988;35(9):653–61.
Peyton PJ. Pulmonary carbon dioxide elimination for cardiac output monitoring in peri-operative and critical care patients: history and current status. J Healthc Eng. 2013;4(2):203–22. doi:10.1260/2040-2295.4.2.203.
Jaffe MB. Partial CO2 rebreathing cardiac output—operating principles of the NICO system. J Clin Monit Comput. 1999;15(6):387–401.
Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK. Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth Analg. 2010;110(3):799–811. doi:10.1213/ANE.0b013e3181cc885a.
Acknowledgement
The faculty at the Hedenstierna laboratory in Uppsala, Sweden.
Author contributions
TS, TÖ, CHS, FSS, MH, AO and HB: Study design, data collection and analysis. TS, CHS, HB: Drafting and revision of the manuscript. ER: Data collection and revision of manuscript. MW: Study design and revision of manuscript. AO, MH, FSS: Critical revision of the manuscript.
Funding
This project is a collaboration between Karolinska Institutet and Maquet Critical Care AB. The work was supported by grants from Maquet Critical Care AB, the regional agreement on medical training and research (ALF) between Stockholm County Council and the Karolinska Institute and the HMT project (Health, Medicine and Technology), a collaboration project between the Stockholm County Council and the Royal Institute of Technology (Grant Nos: 20140430, 20150910).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Magnus Hallbäck and Mats Wallin are employed at Maquet Critical Care AB (MCC). Fernando Suarez Sipmann performs consultant activities for MCC. Håkan Björne and Caroline Hällsjö Sander has received grants for research from MCC. Thorir Sigmundsson, Tomas Öhman, Eider Redondo, Anders Oldner declare no conflict of interest.
Ethical approval
The study was approved by the Uppsala animal research ethical committee (nr. C 47/15) and performed at the Hedenstierna laboratory in Uppsala University, Sweden. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sigmundsson, T.S., Öhman, T., Hallbäck, M. et al. Performance of a capnodynamic method estimating effective pulmonary blood flow during transient and sustained hypercapnia. J Clin Monit Comput 32, 311–319 (2018). https://doi.org/10.1007/s10877-017-0021-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-017-0021-3