Abstract
Despite major advances in anesthesia management and developments in anesthetic agents, postoperative sleep disturbances remain dissatisfactory for many patients. We hypothesized that propofol might have a subtle influence on sleep after thyroidectomy compared to sevoflurane. A randomized, single-blinded, controlled trial was conducted at the First Hospital of China Medical University from October 2014 to October 2015. One hundred and twenty-four patients undergoing thyroidectomy were enrolled and received sevoflurane (sevoflurane group) or propofol (propofol group) as anesthesia maintenance. Major assessments were made during the operation (different types of anesthetic management) and on the first postoperative night (sleep status). The primary outcome was postoperative sleep status, measured by the BIS-Vista monitor on the first night after surgery between propofol and sevoflurane groups. A total of 105 patients (79 women, 26 men; mean age 49 years; range 18–65 years) were included in the final study sample. All patients in both groups showed one of the five sleep patterns classified by this trial. The BIS-area under the curve was decreased, the sleep efficiency index was significantly increased, and the durations of postoperative sleep and sleep stage N3 were increased by 110.5 and 36.5 min per patient, respectively, in the propofol compared to the sevoflurane group. Propofol might preserve sleep time immediately after thyroidectomy. Clinical Trials.gov identifier: NCT 02146976.
Similar content being viewed by others
1 Introduction
Despite major advances in anesthesia management and developments in anesthetic agents, postoperative sleep disturbances remain dissatisfactory for many patients [1, 2]. Sleep disturbances are common among patients in the intensive care unit or on the regular postoperative ward and may be associated with postoperative delirium. Although a previous report studied the implementation of various nonpharmacological interventions, consisting of environmental noise and light [3], in this paper, we evaluated the impacts of the most commonly used anesthetic agents, propofol versus sevoflurane.
Sleep disturbances were found after both propofol-remifentanil and sevoflurane anesthesia [4]. Compared to propofol-remifentanil, sevoflurane was associated with less impairment of postoperative sleep in the first weeks after surgery; however, this conclusion was made on the basis of sleep measurements by subjective sleep dairy. In another study, the incidence of dreaming was significantly higher in the sevoflurane anesthesia group compared to the propofol group [5], although the exact mechanism for this difference was not explained. Rapid eye movement sleep (REM) rebound after exposure to sevoflurane suggests that sevoflurane does not fully substitute for natural sleep [6]. Nevertheless, this result was found only in a mouse model.
The standard approach for sleep tests is to record polysomnograph variables by using a computerized 16-channel recording system [7]. Polysomnographic signals were divided into 30-s epochs and classified as one of the following sleep stages: wakefulness, N1, N2, N3, or R (REM) sleep [8, 9]. However, it is difficult for clinics and patients to accept polysomnographic measurement of postoperative sleep quality. The bispectral index (BIS) technique was developed in the 1990s from a closed algorithm to monitor brain activity in response to different combinations of anesthetics [10]. More recently, studies have suggested that BIS monitors could provide a useful measure of natural physiological sleep depth [9, 11, 12].
We hypothesized that propofol might preserve sleep architecture immediately after surgery. We performed a randomized and controlled clinical trial (RCT) to evaluate the effects of propopol or sevoflurane anesthesia maintenance on the sleep status during the first postoperative night, as monitored by BIS, in patients after thyroidectomy.
2 Materials and methods
2.1 Study design
This RCT was designed to examine the effects of propofol or sevoflurane anesthesia maintenance on postoperative sleep in patients after thyroidectomy. Major assessments were made during the operation and on the first postoperative night. We followed the Consolidated Standards of Reporting Trials (CONSORT) recommendations in designing and reporting the findings of our study. The trial was approved by the Ethics Committee of the First Hospital of China Medical University (protocol no. 20140522, Chairman Prof. Jian Kang, March 10, 2014) and was registered with the Clinical Trials Registry (NCT 02146976). All participants provided written informed consent in accordance with the Declaration of Helsinki.
2.2 Patients
One hundred and twenty-four patients undergoing elective thyroidectomy were enrolled in this study from October 2014 to October 2015 at the First Hospital of China Medical University. Patients were included in the study if they were ethnic Chinese, between 18 and 65 years old, American Society of Anesthesiologists (ASA) physical status I or II, and undergoing thyroidectomy for thyroid tumour. Patients were excluded if they had a body mass index exceeding 30 kg/m2, had a Pittsburgh Sleep Quality Index (PSQI) global scores higher than 6, had an anesthesia time longer than 4 h, had a history of cardiovascular, neurological, or psychiatric disease, had cervical lymph node metastasis, were treated with sedative and analgesics during the postoperative BIS-Vista monitoring period, were allergic to drugs used in the study, or had been diagnosed with hyperthyroidism or hypothyroidism.
2.3 Randomization and masking
All patients requiring thyroidectomy were randomly assigned via a computer-generated sequence with sealed envelopes to one of two groups. In the control group (propofol group), general anesthesia was maintained with propofol and remifentynil. In the sevoflurane group, general anesthesia was maintained with sevoflurane and remifentynil. Treatment allocation was revealed by opening the envelope on the morning of surgery. Anesthesiologists who participated in anesthesia management were not allowed to report study details to the staff who were collecting data. All patients and staff involved in postoperative data collection and analysis were blinded to group allocation. The trial was monitored by an independent data and safety monitoring organization. Group allocation was not revealed until the final statistical analysis was completed.
2.4 General anesthesia induction procedure
All operations involved in the study were completed between 08:00 am and 12:00 noon by the same group of surgeons. Upon arrival in the operating room, electrocardiography was initiated. Heart rate (HR) and mean arterial blood pressure (MAP) were continuously monitored. Arterial oxygen saturation was monitored by pulse oximetry. Inspired oxygen fraction (FiO2) and end-tidal sevoflurane and CO2 levels were measured. The BIS score was monitored with a BIS-Vista monitor (Aspect Medical Systems, Norwood, MA, USA).
After preoxygenation with O2 100% for 3 min, general anesthesia was induced with 0.04 mg/kg midazolam iv, 4 μg/kg fentanyl iv, 2–2.5 mg/kg propofol iv, and 0.2 mg/kg cisatracurium iv. The patient’s lung was ventilated with intermittent positive pressure. After intubation, tidal volume was adjusted to 6–8 ml/kg, and the ventilator rate was adjusted to maintain the end-tidal CO2 at 35–45 mmHg.
2.5 Intervention
For maintenance of sevoflurane anesthesia, the mixture of sevoflurane (Baxter Healthcare, Guayama, Puerto Rico) and air-oxygen was adjusted to maintain an end-tidal sevoflurane concentration of 2–2.5% and an FiO2 of 50%. For maintenance of propofol anesthesia, total intravenous anesthesia was administered by using propofol (Fresenius Kabi, Austria GmbH) at a plasma target concentration (Marsh pharmacokinetic model software; Injectmat ATIV Agilia TCI pump, Fresenius Kabi AG, Bad Homburg, Germany) of 2.5–4 μg/ml and FiO2 of 50%. Remifentanil (0.2–0.5 μg/kg/min) was given to all patients during the operation. Additional cisatracurium (0.05 mg/kg) and fentanyl (30–50 μg at the end of surgery) were used as needed, in accordance with the clinical requirements. Ondansetron (5 mg) was used for prevention of postoperative nausea and vomiting. All cases of anesthesia were finished by one anesthesiologist.
During anesthesia, the BIS was maintained between 40 and 60. Bradycardia (HR <50 bpm) and hypotension (MAP < 20% of baseline) were treated with supplemental doses of atropine (0.5–1.0 mg) and ephedrine (5–10 mg), respectively. Tachycardia (HR >90 bpm) and hypertension (MAP >20% of baseline) were treated with esmolol (5–10 mg) and urapidil (10–25 mg), respectively. At the end of surgery, patient was extubated after return of adequately spontaneous respiration. Neuromuscular blockade was allowed to dissipate spontaneously. The patient was then transferred to the regular postanesthesia care unit (PACU). No additional opioids were given to patients in the PACU. Each patient was in a private room when they were transferred to the ward. All family members who were present were aware of the rules of trial.
2.6 Measures
All patients underwent assessment with the PSQI one day before the operation [13]. The PSQI differentiates between good sleepers (PSQI global score <6) and poor sleepers (PSQI global score ≥6) with high sensitivity and specificity. Intraoperative blood pressure, HR, administered fluids and drugs, and anesthesia duration were recorded. Postoperative pain scores were assessed with an 11-point visual analogue scale (0 = no pain and 10 = worst pain imaginable) on the first postoperative morning after BIS monitoring was completed.
Postoperative nocturnal sleep was evaluated with the BIS-Vista monitor, which is an electroencephalography (EEG)-based signal-processing technique that has been used to evaluate sleep in patients in the intensive care unit (ICU) [14] and in sleep-deprived anesthesiologists [12]. Raw EEG signals were band-pass filtered to 0.2–30 Hz and processed in real time by using Vista application revision 3.20 and Vista platform revision 2.03. BIS recordings were started after verifying that the signal quality index was greater than 95% and the electrode impedance was less than 5 kΩ. With the smoothing window set at 15 s, EEG variables were collected through a USB-A port and stored on a laptop computer. Collected data were analyzed in the off-line mode. BIS data were recorded in 5-second intervals and downloaded onto a personal computer.
Four outcome measures were used in this study: duration of sleep, sleep efficiency index (SEI), area under the curve (AUC), and stage N3 sleep [14]. Sleep was defined as a BIS below 80 [15]. A cutoff of BIS <55, with a sensitivity of 87% and specificity of 93%, was identified as stage N3 in this trial [11]. Duration of sleep was defined as the duration of all BIS data below 80 in the 12 h of monitoring (from 18:00 pm to 06:00 am). SEI was defined as the ratio of a patient’s total sleep time over the time available for “nocturnal” sleep (12 h). BIS-AUC was calculated by using the trapezoidal rule, which uses trapeziums to approximate the region under a curve and calculate its area (GraphPad Prism version 5.01).
According to previous studies,[12, 16, 17] we classified the sleep stages of patients on the basis of their BIS results. Patients were classified as being “awake” if their BIS exceeded 80, in “light sleep” if the BIS was between 60 and 80, and in “SWS” if the BIS was below 60.
2.7 Study outcomes
Primary outcome was the postoperative sleep status, which was measured by the BIS-Vista monitor on the first night after surgery between propofol and sevoflurane groups.
2.8 Statistical analysis
Sample size was calculated according to differences in the BIS-SEI in our pilot study. The calculation [14, 18] assumed an α of 0.05, power of 0.8, minimum detectable difference in SEI of 0.053, and standard deviation (SD) of 0.1. The calculated sample size was 56 patients.
Statistical analysis was performed with the SPSS for Windows software package, version 18 (SPSS, Inc., Chicago, IL). Data are presented as the mean ± SD, absolute values, or median. Before statistical testing, the normality of the distribution of each continuous variable was analyzed by the Kolmogorov–Smirnov test. Continuous data were analyzed with the independent t test, or Mann–Whitney U test. Categorical data were described as the frequency or percentage and were analyzed by the Chi square test.
3 Results
3.1 Patient characteristics
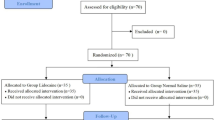
Of the 124 potential patients assessed for eligibility, 12 patients were excluded; thus, 112 patients were randomly assigned to the two groups. Two patients were lost to follow-up, including 1 patients in the propofol group because of BIS equipment error and 1 patients in the sevoflurane group who refused to undergo BIS (Fig. 1). A total of 105 patients (79 women, 26 men; mean age 49 years; range 18–65 years; 101 patients undergoing partial thyroidectomy and four patients undergoing total thyroidectomy) were included in the final study sample. Table 1 reports the results of the demographic and preoperative characteristics of patients in the two groups. There was no significant difference in these characteristics between the two groups.
3.2 Sleep patterns of patients who underwent thyroidectomy on the first postoperative night
Using the identified sleep stages, we classified the sleep patterns of patients as follows:
-
1.
Cycle sleep (Fig. 2a): 30- to 90-minute cycles of alternating light sleep and SWS;
-
2.
Nap (Fig. 2b): Short SWS phase (10–20 min) and awake quickly (BIS quickly returned to >80);
-
3.
Light sleep (Fig. 2c): Hours of light sleep without SWS;
-
4.
Mixed sleep (Fig. 2d): Mixture of the three types of sleep described above; and
-
5.
No sleep (Fig. 2e): BIS exceeded 80 during the whole monitoring period.
All patients in both groups showed one of the five sleep patterns classified above. Compared to the sevoflurane group, 11 more patients in the propofol group had the Cycle Sleep pattern (Table 2). We did not observe the same BIS values for REM sleep as have been reported in patients of the ICU, in healthy volunteers, and in sleep-deprived anesthesiologists [12, 16, 17].
3.3 BIS-AUC, BIS-SEI, stage N3 sleep and duration of sleep during the entire nocturnal sleep period
The BIS-SEI value was increased in the propofol compared to the sevofluorane group, with higher SEI values indicating “better” sleep [14] (Table 3). Furthermore, the durations of stage N3 sleep and total postoperative sleep were significantly increased by 36.5 and 110.5 min, respectively, in the propofol group compared to the sevoflurane group. The BIS-AUC in the propofol group was lower than that in the sevoflurane group, indicating “better” sleep in the propofol group (Table 3).
4 Discussion
The results of this study suggest that patients who undergo anesthesia maintenance with propofol may have better preservation of sleep compared to patients treated with sevoflurane. There are several reasons we chose patients undergoing thyroidectomy for this trial. The study design required a minor surgery that could be completed with both inhalation anesthesia and total intravenous anesthesia, but without sedative or analgesic use during the postoperative BIS-Vista monitoring period.
Bispectral analysis was used to process the EEG signal. This technique was developed in the 1990s from a closed algorithm to monitor brain activity in response to different combinations of anesthetics [10]. However, there have been conflicting results regarding the ability of the BIS monitor to detect sleep stages [19]. Sleigh et al [16] suggested that the BIS monitor can measure the depth of sleep, based on data from five volunteers obtained with the Aspect A-1000 BIS monitor. The same group of researchers did a second study in an ICU, and concluded that almost no ICU patients had normal sleep [17]. Nieuwenhuijs et al [20] did a validation study of the BIS A2000 monitor to a full polysomnogram in 10 patients, and concluded neither the BIS nor the spectral edge frequency could reliably indicate sleep stages. In 2011, Dahaba et al [12] evaluated the bilateral BIS with a BIS-Vista monitor as a tool for measuring sleep in 10 healthy, sleep-deprived anesthesiologists. They demonstrated the ability of the BIS-Vista monitor to detect classical sleep stages.
We obtained satisfactory results in two other clinical trials with the same methodology of the BIS-Vista monitor [21, 22]. On the first night after surgery with general or regional anesthesia with sedative, we could detect differences of sleep status among patients undergoing different anesthesia managements utilizing the BIS-Vista monitor. We speculate that that BIS-Vista monitor did not detect classical sleep stages in ICU or postoperative patients due to reasons other than the BIS-Vista monitoring method per se, but due to the changes in sleep architecture and sleep quality of the patients. Considering the effects of sedatives and analgesics on sleep architecture and sleep quality, we excluded patients who were treated with those drugs. Sleep patterns can be severely disturbed for several nights after surgery, with patients demonstrating marked decreases in the total sleep time, SWS duration, and REM sleep. These features are associated with frequent sleep arousals [23–30]. In a study of 12 healthy young men, the results confirmed that the BIS was highly correlated with the hypnogram results, showing a decrease as sleep deepened and an increase during REM sleep [9]. Given that the sensitivity and specificity of BIS to identify stage N2 sleep (BIS <73) were only 75 and 64%, respectively, in young adults [11], we also identified stage N3 sleep (BIS <55) of both groups in our trial to make the results more closely resemble the polysomnography results.
Although we could not identify REM sleep in our research with the BIS-Vista monitor, we were able to classify the first postoperative sleep pattern into five types. All patients undergoing thyroidectomy with anesthesia maintenance by propofol or sevoflurane had one of these five sleep patterns. Similar to the research results mentioned above, our patients suffered marked decreases in total sleep time and SWS duration, and demonstrated no REM sleep. Interestingly, we found that patients may have light sleep during the whole postoperative night (Fig. 2c) after anesthesia with propofol or sevoflurane. Anesthetic and sedative agents themselves are also known to have immunomodulatory activities [31]. Propofol anesthesia attenuates surgery-induced immune suppression, [32, 33] and sevoflurane decreases the T helpe ratio in vivo [33, 34]. We speculate that propofol may preserve the postoperative sleep by immune protect during the operation. More careful study should be done to clarify the exact mechanism underlying this finding.
This study has several limitations. The main deficiency was the lack of validation to a full polysomnogram. We also need to observe the postoperative sleep disturbance for more days after the operation. Finally, our study included fewer male than female patients, because the incidence of thyroid tumor is higher in females [35]. Considering these limitations, we concluded that propofol might preserve sleep time immediately after thyroidectomy.
Change history
16 June 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10877-023-01045-x
References
Kain ZN, Caldwell-Andrews AA. Sleeping characteristics of adults undergoing outpatient elective surgery: a cohort study. J Clin Anesth. 2003;15(7):505–9.
Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18(3):253–62. doi:10.1016/j.smrv.2013.07.002.
Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540–9. doi:10.1111/anae.12638.
Steinmetz J, Holm-Knudsen R, Eriksen K, Marxen D, Rasmussen LS. Quality differences in postoperative sleep between propofol-remifentanil and sevoflurane anesthesia in infants. Anesth Analg. 2007;104(4):779–83. doi:10.1213/01.ane.0000255694.00651.5b.
Xu GH, Liu XS, Yu FQ, Gu EW, Zhang J, Royse AG, Wang K. Dreaming during sevoflurane or propofol short-term sedation: a randomised controlled trial. Anaesth Intensive Care. 2012;40(3):505–10.
Pick J, Chen Y, Moore JT, Sun Y, Wyner AJ, Friedman EB, Kelz MB. Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology. 2011;115(4):702–12. doi:10.1097/ALN.0b013e31822ddd72.
Kales A, Kales J. Evaluation, diagnosis, and treatment of clinical conditions related to sleep. Jama. 1970;213(13):2229–35.
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172.
Gimenez S, Romero S, Alonso JF, Mananas MA, Pujol A, Baxarias P, Antonijoan RM. Monitoring sleep depth: analysis of bispectral index (BIS) based on polysomnographic recordings and sleep deprivation. J Clin Monit Comput. 2015. doi:10.1007/s10877-015-9805-5.
Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89(4):980–1002.
Benissa MR, Khirani S, Hartley S, Adala A, Ramirez A, Fernandez-Bolanos M, Quera-Salva MA, Fauroux B. Utility of the bispectral index for assessing natural physiological sleep stages in children and young adults. J Clin Monit Comput. 2015. doi:10.1007/s10877-015-9800-x.
Dahaba AA, Xue JX, Xu GX, Liu QH, Metzler H. Bilateral Bispectral Index (BIS)-Vista as a measure of physiologic sleep in sleep-deprived anesthesiologists. Minerva Anestesiol. 2011;77(4):388–93.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12(2):R52. doi:10.1186/cc6871.
Tung A, Lynch JP, Roizen MF. Use of the BIS monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17(1):37–42.
Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88(3):659–61.
Nicholson T, Patel J, Sleigh JW. Sleep patterns in intensive care unit patients: a study using the bispectral index. Crit Care Resusc. 2001;3(2):86–91.
Lerman J. Study design in clinical research: sample size estimation and power analysis. Can J Anaesth. 1996;43(2):184–91. doi:10.1007/bf03011261.
Nieuwenhuijs DJ. Processed EEG in natural sleep. Best Pract Res Clin Anaesthesiol. 2006;20(1):49–56.
Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94(1):125–9. (table of contents).
Tan WF, Guo B, Ma H, Li XQ, Fang B, Lv HW. Changes in postoperative night bispectral index of patients undergoing thoracic surgery with different types of anaesthesia management: a randomized controlled trial. Clin Exp Pharmacol Physiol. 2016;43(3):304–11. doi:10.1111/1440-1681.12530.
Tan WF, Miao EY, Jin F, Ma H, Lu HW. Changes in First Postoperative Night Bispectral Index After Daytime Sedation Induced by Dexmedetomidine or Midazolam Under Regional Anesthesia: A Randomized Controlled Trial. Reg Anesth Pain Med. 2016;41(3):380–6. doi:10.1097/aap.0000000000000370.
Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109(5):769–75. doi:10.1093/bja/aes252.
Rosenberg-Adamsen S, Kehlet H, Dodds C, Rosenberg J. Postoperative sleep disturbances: mechanisms and clinical implications. Br J Anaesth. 1996;76(4):552–9.
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. The Lancet. 2006;367(9522):1618–25. doi:10.1016/s0140-6736(06)68700-x.
Rosenberg J, Wildschiodtz G, Pedersen MH, von Jessen F, Kehlet H. Late postoperative nocturnal episodic hypoxaemia and associated sleep pattern. Br J Anaesth. 1994;72(2):145–50.
Rosenberg-Adamsen S, Skarbye M, Wildschiodtz G, Kehlet H, Rosenberg J. Sleep after laparoscopic cholecystectomy. Br J Anaesth. 1996;77(5):572–5.
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep. 2001;24(1):39–44.
Gogenur I, Wildschiotz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100(1):45–9. doi:10.1093/bja/aem340.
Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W. Postoperative changes in sleep-disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):287–98. doi:10.1097/aln.0000000000000040.
Hunter JD. Effects of anaesthesia on the human immune system. Hosp Med. 1999, 60(9):658–663.
Kelbel I, Weiss M. Anaesthetics and immune function. Curr Opin Anaesthesiol. 2001;14(6):685–91.
Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, Shingu K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59(10):954–9. doi:10.1111/j.1365-2044.2004.03837.x.
Ji FH, Wang YL, Yang JP. Effects of propofol anesthesia and sevoflurane anesthesia on the differentiation of human T-helper cells during surgery. Chin Med J (Engl). 2011;124(4):525–9.
Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, Liu Y. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit. 2013;19:49–53.
Acknowledgements
We would like to thank Dr. Hao Zhang and Dr. Ping Zhang (Department of Thyroid Surgery, The First Hospital of China Medical University) for their help in the enrolment of patients.
Funding
This work was funded by: the natural science foundation of Liaoning Province (2014021035) to Wen-fei Tan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest.
About this article
Cite this article
Tan, Wf., Wang, Zl., Ma, H. et al. RETRACTED ARTICLE: Changes in the first postoperative night bispectral index of patients after thyroidectomy with different types of primary anesthetic management: a randomized controlled trial. J Clin Monit Comput 32, 165–172 (2018). https://doi.org/10.1007/s10877-016-9974-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9974-x