Abstract
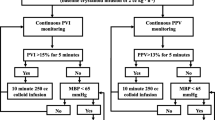
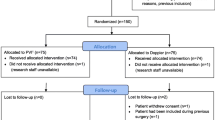
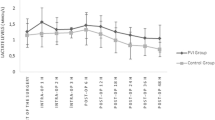
Pleth variability index (PVI), a noninvasive dynamic indicator of fluid responsiveness has been demonstrated to be useful in the management of the patients with goal directed fluid therapy under general anesthesia, but whether PVI can be used to optimize fluid management under combined general and epidural anesthesia (GEN–EPI) remains to be elucidated. The aim of our study was to explore the impact of PVI as a goal-directed fluid therapy parameter on the tissue perfusion for patients with GEN–EPI. Thirty ASA I–II patients scheduled for major abdominal surgeries under GEN–EPI were randomized into PVI-directed fluid management group (PVI group) and non PVI-directed fluid management group (control group). 2 mL/kg/h crystalloid fluid infusion was maintained in PVI group, once PVI > 13 %, a 250 mL colloid or crystalloid was rapidly infused. 4–8 mL/kg/h crystalloid fluid infusion was maintained in control group, and quick fluid infusion was initiated if mean arterial blood pressure (BP) < 65 mmHg. Small doses of norepinephrine were given to keep mean arterial BP above 65 mmHg as needed in both groups. Perioperative lactate levels, hemodynamic changes were recorded individually. The total amount of intraoperative fluids, the amount of crystalloid fluid and the first hour blood lactate levels during surgery were significantly lower in PVI than control group, P < 0.05. PVI-based goal-directed fluid management can reduce the intraoperative fluid amount and blood lactate levels in patients under GEN–EPI, especially the crystalloid. Furthermore, the first hour following GEN–EPI might be the critical period for anesthesiologist to optimize the fluid management.



Similar content being viewed by others
References
Rawal N. Combined regional and general anaesthesia. Curr Opin Anaesthesiol. 2000;13(5):531–7.
Merquiol F, Montelimard AS, Nourissat A, Molliex S, Zufferey PJ. Cervical epidural anesthesia is associated with increased cancer-free survival in laryngeal and hypopharyngeal cancer surgery: a retrospective propensity-matched analysis. Reg Anesth Pain Med. 2013;. doi:10.1097/AAP.0b013e31829cc3fb.
Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, Collins KS, Group MATS. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276–82. doi:10.1016/S0140-6736(02)08266-1.
Dunet F, Pfister C, Deghmani M, Meunier Y, Demeilliers-Pfister G, Grise P. Clinical results of combined epidural and general anesthesia procedure in radical prostatectomy management. Can J Urol. 2004;11(2):2200–4.
El-Morsy GZ, El-Deeb A. The outcome of thoracic epidural anesthesia in elderly patients undergoing coronary artery bypass graft surgery. Saudi J Anaesth. 2012;6(1):16–21. doi:10.4103/1658-354X.93048.
Zhao W, Zhou R, Zhou LP, Li CH. The hemodynamic effects during thoracic epidural anesthesia combined with general anesthesia in patients undergoing major abdominal operations. Zhonghua wai ke za zhi [Chin J Surg]. 2009;47(11):849–52.
Gurses E, Berk D, Sungurtekin H, Mete A, Serin S. Effects of high thoracic epidural anesthesia on mixed venous oxygen saturation in coronary artery bypass grafting surgery. Med Sci Monit Int Med J Exp Clin Res. 2013;19:222–9. doi:10.12659/MSM.883861.
Shin S, Bai SJ, Rha KH, So Y, Oh YJ. The effects of combined epidural and general anesthesia on the autonomic nervous system and bioavailability of nitric oxide in patients undergoing laparoscopic pelvic surgery. Surg Endosc. 2013;27(3):918–26. doi:10.1007/s00464-012-2536-5.
Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth. 2002;14(4):252–6.
Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso E, Ording H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid T. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8. doi:10.1097/01.sla.0000094387.50865.23.
Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32(3):691–9.
Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: functional haemodynamic parameters including the Respiratory Systolic Variation Test and static preload indicators. Br J Anaesth. 2005;95(6):746–55. doi:10.1093/bja/aei262.
Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense*. Crit Care Med. 2013;41(7):1774–81. doi:10.1097/CCM.0b013e31828a25fd.
Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006;103(6):1478–84. doi:10.1213/01.ane.0000246811.88524.75.
Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108(2):513–7. doi:10.1213/ane.0b013e318192a36b.
Cannesson M, Desebbe O, Rosamel P, Delannoy B, Robin J, Bastien O, Lehot JJ. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200–6. doi:10.1093/bja/aen133.
Sandroni C, Cavallaro F, Marano C, Falcone C, De Santis P, Antonelli M. Accuracy of plethysmographic indices as predictors of fluid responsiveness in mechanically ventilated adults: a systematic review and meta-analysis. Intensive Care Med. 2012;38(9):1429–37. doi:10.1007/s00134-012-2621-1.
Vos JJ, Kalmar AF, Struys MM, Wietasch JK, Hendriks HG, Scheeren TW. Comparison of arterial pressure and plethysmographic waveform-based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth. 2013;110(6):940–6. doi:10.1093/bja/aes508.
Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111(4):910–4. doi:10.1213/ANE.0b013e3181eb624f.
Valenza F, Aletti G, Fossali T, Chevallard G, Sacconi F, Irace M, Gattinoni L. Lactate as a marker of energy failure in critically ill patients: hypothesis. Crit Care. 2005;9(6):588–93. doi:10.1186/cc3818.
Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi:10.1186/2110-5820-3-12.
Desebbe O, Cannesson M. Using ventilation-induced plethysmographic variations to optimize patient fluid status. Curr Opin Anaesthesiol. 2008;21(6):772–8. doi:10.1097/ACO.0b013e32831504ca.
Fu Q, Mi WD, Zhang H. Stroke volume variation and pleth variability index to predict fluid responsiveness during resection of primary retroperitoneal tumors in Hans Chinese. Biosci Trends. 2012;6(1):38–43. doi:10.5582/bst.2012.v6.1.38.
Kabon B, Fleischmann E, Treschan T, Taguchi A, Kapral S, Kurz A. Thoracic epidural anesthesia increases tissue oxygenation during major abdominal surgery. Anesth Analg. 2003;97(6):1812–7.
Treschan TA, Taguchi A, Ali SZ, Sharma N, Kabon B, Sessler DI, Kurz A. The effects of epidural and general anesthesia on tissue oxygenation. Anesth Analg. 2003;96(6):1553–7 Table of contents.
Bettesworth J, Bhalla T, Barry N, Tobias JD. Changes in tissue oxygenation following caudal epidural blockade in infants and children. Paediatr Anaesth. 2012. doi:10.1111/j.1460-9592.2012.03925.x.
Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. The British journal of surgery. 2006;93(9):1069–76. doi:10.1002/bjs.5454.
Monnet X, Guerin L, Jozwiak M, Bataille A, Julien F, Richard C, Teboul JL. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110(2):207–13. doi:10.1093/bja/aes373.
Biais M, Cottenceau V, Petit L, Masson F, Cochard JF, Sztark F. Impact of norepinephrine on the relationship between pleth variability index and pulse pressure variations in ICU adult patients. Crit Care. 2011;15(4):R168. doi:10.1186/cc10310.
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (81271263 to J.J.Z), Shanghai Pujiang Talent Program from Science and Technology Commission of Shanghai Municipality, China (11PJ1408000 to J.J.Z), and Medical Climbing Program from Songjiang Health Bureau, China (2011PD15 to J.J.Z).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Y., Dong, J., Xu, Z. et al. Pleth variability index-directed fluid management in abdominal surgery under combined general and epidural anesthesia. J Clin Monit Comput 29, 47–52 (2015). https://doi.org/10.1007/s10877-014-9567-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9567-5