Abstract
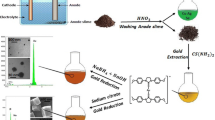
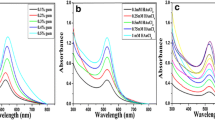
The present investigation reports the first-time successful synthesis of AuNPs using a new precursor salt of Au(III) acetate through USP. An aqueous solution of this salt was prepared with very limited solubility with H2O. HCl and HNO3 were then added separately to increase the solubility, resulting in a clear, yellowish solution. This enabled the successful formation of AuNPs with USP. In order to improve AuNPs synthesis, NaOH and Na2CO3 were added into the precursor to increase its pH (6–7). With such approach, it was possible to perform USP synthesis using varying concentrations of [Au] in the precursor. Evaporation and reaction temperatures (100 and 300 °C) of USP were chosen based on detected decomposition temperatures of Au(III) acetate with TGA-DT. TEM confirmed the presence of circular shaped, unagglomerated AuNPs having an Fm-3m space group with diameter range of 15–30 and circularity value range of 0.89–0.92. The UV–Vis spectroscopy showed absorbance peaks at 528 and 532 nm. ICP-MS indicated the highest concentration of AuNPs, 79 ppm, by the precursor with the lower initial concentration of [Au]. This could be due to the smallest sedimentation and turbulent losses of larger AuNPs in transport tubes and reaction USP zones.






Similar content being viewed by others
Abbreviations
- AuNPs:
-
Gold nanoparticles
- USP:
-
Ultrasonic spray pyrolysis
- Au(III) acetate:
-
Gold(III) acetate
- TGA-DT:
-
Thermal gravimetric analysis—differential thermal
- MOx :
-
Metal oxide powder
- TEM:
-
Transmission electron microscopy
- UV–Vis:
-
Ultraviolet visible
- SPR:
-
Surface plasmon resonance
- PEG:
-
Polyethylene glycol
- PVP:
-
Polyvinylpyrrolidone
- BSA:
-
Bovine serum albumin
- wt%:
-
Weight percent
- RES:
-
Reticuloendothelial system
- EDX:
-
Energy-dispersive X-ray spectroscopy
- DLS:
-
Dynamic light scattering
- FCC:
-
Face centered cubic
- ICP-OES:
-
Optical emission spectroscopy with inductively coupled plasma mass
- FTIR:
-
Fourier transform infrared spectroscopy
- SD:
-
Standard deviation
- fg:
-
Femto gram (10−15)
References
B. Sepúlveda, P. C. Angelomé, L. M. Lechuga, and L. M. Liz-Marzán (2009). Nano Today 4, (3), 244–251.
K. M. Mayer and J. H. Hafner (2011). Chemical Reviews 111, (6), 3828–3857.
K. Saha, S. S. Agasti, C. Kim, X. Li, and V. M. Rotello (2012). Chemical Reviews 112, (5), 2739–2779.
J. Z. Zhang Optical Properties and Spectroscopy of Nanomaterials, 1st ed (World Scientific Publishing Company, Singapore, 2009).
P. Alivisatos (2004). Nature Biotechnology 22, 47–52.
J. L. West and N. J. Halas (2000). Current Opinion in Biotechnology 11, 215–217.
I. K. Ding, J. Zhu, W. Cai, et al. (2011). Advanced Energy Materials 1, (1), 52–57.
D. Wu, X. Xu, and X. Liu (2008). The Journal of Chemical Physics 129, 074313.
A. Lahde, I. Koshevoy, T. Karhunen, T. Torvela, T. A. Pakkanen, and J. Jokiniemi (2014). Journal of Nanoparticle Research. doi:10.1007/s11051-014-2716-4.
O. Masala and R. Seshadri (2004). Annual Review of Materials Research 34, 41–81.
M. Brust and C. J. Kiely (2002). Colloids and Surfaces 202, 175–186.
Y. Yin, C. Erdonmez, S. Aloni, and A. P. Alivisatos (2006). Journal of the American Chemical Society 128, 12671–12673.
N. Bao, L. Shen, Y. Wang, P. Padhan, and A. Gupta (2007). Journal of the American Chemical Society 129, 12374–12375.
H. Hiramatsu and F. E. Osterloh (2004). Chemistry of Materials 16, 2509–2511.
S. Sun, B. C. Murray, D. Weller, L. Folks, and A. Moser (2000). Science 287, 1989–1992.
S. Sun and H. Zeng (2002). Journal of the American Chemical Society 124, 8204–8205.
M. Haruta, S. Tsubota, T. Kobayashi, H. Kageyama, M. J. Genet, and B. Delmon (1993). Journal of Catalysis 144, 175–192.
S. Ivanova, V. Pitchon, Y. Zimmermann, and C. Petit (2006). Applied Catalysis, A: General 298, 57–64.
M. Bowker, A. Nuhu, and J. Soares (2007). Catalysis Today 122, 245–247.
J. D. Lessard, I. Valsamakis, and M. Flytzani-Stephanopoulos (2012). Chemical Communications 48, 4857–4859.
A. Hugon, N. E. L. Kolli, and C. Louis (2010). Journal of Catalysis 274, 239–250.
X. Lu, H. Y. Tuan, B. A. Korgel, and Y. Xia (2008). Chemistry--A European Journal 14, 1584–1591.
A. Lahde, I. Koshevoy, T. Karhunen, T. Torvela, T. A. Pakkanen, and J. Jokiniemi (2014). Journal of Nanoparticle Research. doi:10.1007/s1105101427164.
M. Garza, I. López, and I. Gómez (2013). Advances in Materials Science and Engineering 916908, 1–5.
S. D. Bakrania, G. K. Rathore, and M. S. Wooldridge (2009). Journal of Thermal Analysis and Calorimetry 95, (1), 117–122.
H. Sakurai, K. Koga, Y. Iizuka, and M. Kiuchia (2013). Applied Catalysis, A: General 462–463, 236–246.
M. T. Htay, Y. Hashimoto, N. Momose, and K. Ito (2009). Journal of Crystal Growth 311, (20), 4499–4504.
M. A. Montero, M. R. G. Chialvo, and A. C. Chialvo (2009). Journal of Materials Chemistry 19, (20), 3276–3280.
U. Alver, T. Kılınç, E. Bacaksiz, and S. Nezir (2007). Materials Chemistry and Physics 106, (2–3), 227–230.
H. Zhang and M. T. Swihart (2007). Chemistry of Materials 19, (6), 1290–1301.
S. E. Skrabalak and K. S. Suslick (2005). Journal of the American Chemical Society 127, (28), 9990–9991.
P. Majerič, R. Rudolf, I. Anžel, J. Bogović, S. Stopić, and B. Friedrich (2015). Materials Technology 49, (1), 75–80.
D. Mott, et al. (2009). Chemistry of Materials 22, 261–271.
D. Mott, J. Galkowski, L. Wang, J. Luo, and J. C. Zhong (2007). Langmuir 23, 5740–5745.
Z. Xu, C. Shen, Y. Hou, H. Gao, and S. Sun (2009). Chemistry of Materials 21, 1778–1780.
S. D. Bakrania, T. A. Miller, C. Perez, and M. S. Wooldridge (2007). Combustion and Flame 148, 76.
S. D. Bakrania, C. Perez, and M. S. Wooldridge (2007). Proceedings of the Combustion Institute 31, (II), 1797–1804.
L. Mangolini, E. Thimsen, and U. Kortshagen (2005). Nano Letters 5, (4), 655.
E. Thimsen and P. Biswas (2005). AIChE Journal 53, (7), 1727.
E. Thimsen, N. Rastgar, and P. Biswas (2005). Journal of Physical Chemistry 112, (11), 4134.
P. Biswas and E. Thimsen Aerosol Measurements, 3rd ed (Wiley-VCH, New York, 2011). (Chapter 33).
R. Rudolf, B. Friedrich, S. Stopic, I. Anzel, S. Tomic, and M. Colic (2012). Journal of Biomaterials Applications 26, 595–612.
J. Dokic, R. Rudolf, S. Tomic, S. Stopic, B. Friedrich, B. Budic, I. Anzel, and M. Colic (2012). Journal of Biomedical Nanotechnology 8, 528–538.
M. Afzal, P. K. Butt, and H. Ahmad (1991). Journal of Thermal Analysis 37, 1015.
S. Stopic, R. Rudolf, J. Bogovic, P. Majeric, M. Colic, S. Tomic, M. Jenko, and B. Friedrich (2013). MTAEC9 47, (5), 557–583.
S. Stopic, B. Friedrich, H. U. Fritsching, K. Raic, Synthesis of metallic nanosized particles by ultrasonic spray pyrolysis, IME Metallurgische Prozesstechnik and Metallrecycling, RWTH Aachen, Germany, 1st ed (Shaker Verlag, 2015).
P. Majeric, D. Jenko, B. Budic, S. Tomic, M. Colic, B. Friedrich, and R. Rudolf (2015). Nanoscience and Nanotechnology Letters 7, 1–10.
P. Majerič, B. Friedrich, and R. Rudolf (2015). Materials Technology 49, (1), 791–796.
R. Rudolf, P. Majeric, S. Tomic, M. Shariq, U. Fercec, B. Budic, B. Friedrich, D. Vucevic, M. Colic, Journal of Nanomaterials (2017). doi:10.1155/2017/9365012.
A. Barreto, L. G. Luis, A. V. Girao, T. Trindade, M. Amadeu, V. M. Soares, and M. Oliveira (2015). Journal of Nanoparticle Research. doi:10.1007/s1105101533020.
S. C. Tsai, Y. L. Song, C. S. Tsai, C. C. Yang, W. Y. Chiu, and H. M. Lin (2004). Journal of Materials Science 39, 3647–3657.
T. Niidome, M. Yamagata, Y. Okamoto, et al. (2006). Journal of Controlled Release 114, (3), 343–347.
D. K. Kim, S. J. Park, J. H. Lee, Y. Y. Jeong, and S. Y. Jon (2007). Journal of the American Chemical Society 129, (24), 7661–7665.
C. J. Liu, C. H. Wang, C. C. Chien, et al. (2008). Nanotechnology 19, 29.
S. K. Seol, D. Kim, S. Jung, W. S. Chang, and J. T. Kim (2013). Journal of Nanomaterials. doi:10.1155/2013/531760.
B. D. Warheit (2008). Toxicological Sciences 101, 183–185.
L. Canesi, C. Ciacci, R. Fabbri, A. Marcomini, G. Pojana, and G. Gallo (2012). Marine Environment Research 76, 16–21.
A. L. Fernandez, A. Fernandez, and J. Blasco (2012). TrAC Trends in Analytical Chemistry 32, 40–59.
T. B. Lee and F. J. Ranville (2012). Journal of Hazardous Materials 213–214, 434–439.
S. Balog, L. R. Lorenzo, A. C. Monnier, M. R. Obiols, B. R. Rothen, P. Schurtenberger, and A. F. Petri (2015). Nanoscale 7, 5991–5997.
Y. Liu, K. M. Shipton, J. Ryan, D. E. Kaufman, S. Franzen, and L. D. Feldheim (2007). Analytical Chemistry 79, 2221–2229.
V. J. Jokerst, T. Lobovkina, N. R. Zare, and S. S. Gambhir (2011). Nanomedicine 6, 715–728.
J. Manson, D. Kumar, B. Meenan, and D. Dixon (2011). Gold Bulletin 44, 99–105.
L. H. T. Nghiem, T. T. Nguyen, E. Fort, P. T. Nguyen, N. M. T. Hoang, Q. T. Nguyen, and N. H. Tran (2012). Advances in Natural Sciences 3, 015002.
C. N. R. Rao, G. U. Kulkarni, P. J. Thomas, and P. P. Edwards (2000). Chemical Society Reviews 29, 27.
H. Bonnemann and R. M. Richards (2001). European Journal of Inorganic Chemistry 1434, 2455–2480.
X. Sun, S. Dong, and E. Wang (2006). Materials Chemistry and Physics 96, 29–33.
N. Srivastava and M. Mukhopadhyay (2015). Journal of Cluster Science. doi:10.1007/s10876-014-0726-0.
N. Saha and S. D. Gupta (2016). Journal of Cluster Science. doi:10.1007/s10876-016-1009-8.
A. Parveen and S. Rao (2015). Journal of Cluster Science. doi:10.1007/s10876-014-0813-2.
T. T. Kodas and M. H. Smith Aerosol Processing of Materials, 1st ed (Wiley-VCH, New York, 1999), pp. 45–74.
J. P. Sylvestre, A. V. Kabashin, E. Sacher, and J. H. T. Luong (2004). Journal of the American Chemical Society. doi:10.1021/ja048678s.
G. Cardenas, V. Saez, and C. Cruzat (2015). Journal of Cluster Science. doi:10.1007/s10876-016-1071-2.
Acknowledgements
The study was supported by the European Union—Erasmus Mundus Action 2 Lot 13 Euphrates Program and Slovenian Research Agency ARRS Slovenia (P2-120 and Martina Program). Many thanks to Dr.Vanja Kokol, Dr. Irena Ban and Mrs. Vera Vivod for helping in the UV–Vis spectroscopy, TGA and FTIR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Shariq, M., Majerič, P., Friedrich, B. et al. Application of Gold(III) Acetate as a New Precursor for the Synthesis of Gold Nanoparticles in PEG Through Ultrasonic Spray Pyrolysis. J Clust Sci 28, 1647–1665 (2017). https://doi.org/10.1007/s10876-017-1178-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1178-0