Abstract
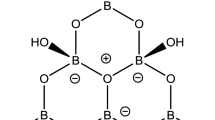
A series of four new clusters with cationic unit [Co4(µ3-OH)(Rdea)2(L–L)4]4+ [R = –CH3 (1 and 2) or –CH2CH2CH2CH3 (3 and 4), L–L = 1,10-phenanthroline or 2,2′-bipyridine] is designed and characterized by elemental, spectroscopic (FTIR, ESI MS, UV–visible), thermal, electrochemical, magnetic, SEM, EDX, PXRD and single crystal X-ray crystallographic techniques. Spectral and single crystal X-ray studies reveal the complexes 1–4 are tetranuclear clusters where the primary aminoalcohol derivative ligand is present in dianionic form i.e., Medea2− or nBudea2−. It is confirmed from the magnetic and bond valence summation data that the four cobalt ions are present in mixed valent states (Co 2+2 –Co 3+2 ), which is further corroborated from the Co–O and Co–N bond lengths in X-ray structure. The bridging two hydroxide ions consolidate the tetranuclear clusters by bonding with three neighboring metal ions and a rare incomplete dicubane core is formed in all the complexes. A supramolecular framework is generated by H-bonding, π–π, CH···π, and H···H interactions. Presence of N-alkyl group and N-heterocyclic chelator facilitates the generation of these non covalent interactions thus stabilizing the framework. Of all the complexes reported here, 1 is found most efficient catalyst for the cobalt-catalyzed aerobic oxidation of neat ethylbenzene and p-xylene.
















Similar content being viewed by others
References
E. G. Mednikov and L. F. Dahl (2008). Small 4, 534.
G. E. Kostakis, S. P. Perlepes, V. A. Blatov, D. M. Proserpio, and A. K. Powell (2012). Coord. Chem. Rev. 256, 1246.
G. Schmid Nanoparticles—From Theory to Application, vol. 19 (Wiley-VCH, Weinheim, 2005), p. 7.
G. Aromi and E. K. Brechin (2006). Struct. Bond. 1, 122.
J. J. Zhang, T. L. Sheng, S. Q. Xia, G. Leibeling, F. Meyer, S. M. Hu, R. B. Fu, S. C. Xiang, and X. T. Wu (2004). Inorg. Chem. 43, 5472.
R. E. P. Winpenny (2001). Adv. Inorg. Chem. 52, 1 (ed. A.G. Sykes).
R. E. P. Winpenny (2002). J. Chem. Soc. Dalton Trans. 1.
O. V. Nesterova, M. V. Kirillova, M. Fátima, C. G. da Silva, R. Bočacd, and A. J. L. Pombeiro (2014). Cryst. Eng. Comm. 16, 775.
R. W. Saalfrank, H. Maid, and A. Scheurer (2008). Angew. Chem. Int. Ed. 47, 8794.
F. H. Allen (2002). Acta Crystallogr. Sect. B Struct. Sci. 58, 380.
T. C. Stamatatos, D. Foguet-Albiol, W. Wernsdorfer, K. A. Abboud, and G. Christou (2011). Chem. Commun. 47, 274.
S. K. Langley, K. J. Berry, B. Moubaraki, and K. S. Murray (2009). Dalton Trans. 973.
M. Murugesu, F. Wernsdorfer, K. A. Abboud, and G. Christou (2005). Angew. Chem. Int. Ed. 44, 892.
B. Moubaraki, K. S. Murray, T. A. Hudson, and R. Robson (2008). Eur. J. Inorg. Chem. 29, 4525.
C. Philouze, G. Blondin, J.-J. Girerd, J. Guilhem, C. Pascard, and D. Lexa (1994). J. Am. Chem. Soc. 116, 8557.
S. Shit, G. Rosair, and S. Mitra (2011). J. Mol. Struct. 79, 991.
S. Wang, K. Folting, W. E. Streib, E. A. Schmitt, J. K. McCusker, D. N. Hendrickson, and G. Christou (1991). Angew. Chem. Int. Ed. 30, 305.
E. S. Lang, R. Stieler, and G. Manzoni de Oliveira (2010). Polyhedron 29, 1760.
S. Mukhopadhyay, R. J. Staples, and W. H. Armstrong (2002). Chem. Commun. 864.
M. L. Kirk, M. K. Chan, W. H. Armstrong, and E. I. Solomon (1992). J. Am. Chem. Soc. 114, 10432.
T. C. Stamatatos, S. Dionyssopoulou, G. Efthymiou, P. Kyritsis, C. P. Raptopoulou, A. Terzis, R. Vicente, A. Escuer, and S. P. Perlepes (2005). Inorg. Chem. 44, 3374.
P. M. Stricklen, E. J. Volcko, and J. G. Verkade (1983). J. Am. Chem. Soc. 105, 2494.
M. Fujita, J. Yazaki, and K. Ogura (1990). J. Am. Chem. Soc. 112, 5645.
M. Shatruk, C. Avendano, and K. R. Dunbar (2009). Prog. Inorg. Chem. 56, 155. (ed. K. D. Karlin).
L. M. Toma, R. Lescouezec, D. Armentano, G. de Munno, and M. Andruh, J. Cano, F. Lloret, and M. Julve (2005). Dalton Trans. 1357.
T. Shiga and H. Oshio (2005). Sci. Technol. Adv. Mater. 6, 565.
A. Caneschi, D. Gatteschi, and R. Sessoli (1991). J. Am. Chem. Soc. 113, 5873.
D. Li, R. Clerac, O. Roubeau, E. Harte, C. Mathoniere, R. Lebris, and S. M. Holmes (2008). J. Am. Chem. Soc. 130, 252.
R. D. Adams and B. Captain (2008). Angew. Chem. Int. Ed. 47, 252.
S. Mukhopadhyay, S. K. Mandal, S. Bhaduri, and W. H. Armstrong (2004). Chem. Rev. 104, 3981.
E. Adman, L. C. Sieker, and L. H. Jensen American Crystallographic Association, Winter Meeting, Symposia: Experimental and theoretical studies of the electron density in crystals and molecules (University of New Mexico, Albuquerque, 1972) (Abstract L2).
B. Schmid, H.-J. Chiu, V. Ramakrishnan, J. B. Howard, and D. C. Rees, in A. Messerschmidt, R. Huber, T. Poulos and K. Wieghardt (eds.), Handbook of Metalloproteins (John Wiley & Sons, Ltd., Chichester, New York, Weinheim, Brisbane, Singapore, Toronto, 2001), p. 1025.
Z. A. Siddiqi, A. Siddique, M. Shahid, M. Khalid, P. K. Sharma, Anjuli, M. Ahmad, S. Kumar, Y. Lan, and A. K. Powell (2013). Dalton Trans. 42, 9513.
D. Gatteschi, R. Sessoli, and A. Cornia (2000). Chem. Commun. 725.
A. K. Powell, S. L. Heath, D. Gatteschi, L. Pardi, R. Sessoli, G. Spina, F. DelGiallo, and F. Pieralli (1995). J. Am. Chem. Soc. 117, 2491.
E. K. Brechin, S. G. Harris, A. Harrison, S. Parsons, A. G. Whittaker, and R. E. P. Winpenny (1997). Chem. Commun. 653.
Z. E. Serna, M. K. Urtiaga, M. G. Barandika, R. Cortes, S. Martin, L. Lezama, M. I. Arriortua, and T. Rojo (2001). Inorg. Chem. 40, 4550.
S. Saha, S. Pal, C. J. Góomez-García, J. M. Clemente-Juan, K. Harms, and H. P. Nayek (2014). Polyhedron 28, 1.
E.-C. Yang, W. Wernsdorfer, S. Hill, R. S. Edwards, M. Nakano, S. Maccagnano, L. N. Zakharov, A. L. Rheingold, G. Christou, and D. N. Hendrickson (2003). Polyhedron 22, 1727.
Z. E. Serna, L. Lezama, M. K. Urtiaga, M. I. Arriortua, M. G. Barandika, R. Cortes, and T. Rojo (2000). Angew. Chem. Int. Ed. 112, 352.
E. Yang, D. N. Hendrickson, W. Wernsdorfer, M. Nakano, L. N. Zakharov, R. D. Sommer, A. L. Rheingold, M. Ledezma-Gairaud, and G. Christou (2002). J. Appl. Phys. 91, 7382.
D. Mandal and D. Ray (2007). Inorg. Chem. Comm. 10, 1202.
W.-X. Zhang, C.-Q. Ma, X.-N. Wang, Z.-G. Yu, Q.-J. Lin, and D.-H. Jiang (1995). Chin. J. Chem. 13, 497.
K. Dhara, J. J. Ratha, M. Manassero, X.-Y. Wang, S. Gao, and P. Banerjee (2007). J. Inorg. Biochem. 95, 101.
J. M. Clemente-Juan, C. Mackiewicz, M. Verelst, F. Dahan, A. Bousseksou, Y. Sanakis, and J. P. Tuchagues (2002). Inorg Chem. 41, 1478.
G. S. Papaefstathiou, A. Escuer, C. P. Raptopoulou, A. Terzis, S. P. Perlepes, and R. Vicente (2001). Eur. J. Inorg. Chem. 2001, 1567.
S. L. Castro, Z. Sun, C. M. Grant, J. C. Bollinger, D. N. Hendrickson, and G. Christou (1998). J. Am. Chem. Soc. 120, 2365.
T. C. Stamatatos, A. K. Boudalis, K. V. Pringouri, C. P. Raptopoulou, A. Terzis, J. Wolowska, E. J. L. McInnes, and S. P. Perlepes (2007). Eur. J. Inorg. Chem. 2007, 5098.
E. Colacio, M. Ghazi, R. Kivekäs, and J. M. Moreno (2000). Inorg. Chem. 39, 2882.
I. A. Ansari, F. Sama, M. Raizada, M. Shahid, M. Ahmad, and Z. A. Siddiqi (2016). New J. Chem. 40, 9840.
T. G. Carrell, S. Cohen, and G. C. Dismukes (2002). J. Mol. Catal. A Chem. 187, 3.
J. A. Ibers and W. C. Hamilton (eds.) International Tables for X-ray Crystallography, vol. IV (Kynoch Press, Birmingham, 1974), p. 71.
SMART & SAINT Software Reference Manuals, Version 6.45 (Bruker Analytical X-ray Systems, Inc., Madison, 2003).
G. M. Sheldrick SADABS, software for empirical absorption correction (University of Göttingen, Göttingen, 2002).
XPREP, 5.1 ed. (Siemens Industrial Automation Inc., Madison, 1995).
G. M. Sheldrick SHELXL97, Program for Crystal Structure Refinement (University of Göttingen, Göttingen, 2008).
A. Altomare, M. C. Burla, M. Camalli, G. L. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. G. Moliterni, G. Polidori, and R. J. Spagna (1999). J. Appl. Crystallogr. 32, 115.
A. L. Spek (2009). Acta Cryst. D65, 148.
W. Geary (1971). Coord. Chem. Rev. 7, 81.
K. Nakamoto Infrared, Raman Spectra of Inorganic and Coordination Compounds (Wiley-Interscience, New York, 1986).
R. P. Sharma, A. Saini, P. Venugopalan, V. Ferretti, F. Spizzo, C. Angeli, and C. J. Calzado (2014). New J. Chem. 38, 274.
Z. G. Li, J. W. Xu, H. Q. Jia, and N. H. Hu (2006). Inorg. Chem. Commun. 9, 969.
N. T. Madhu and P. K. Radhakrishnan (2000). Trans. Met. Chem. 25, 287.
Z. Boulsourani, V. Tangoulis, C. P. Raptopoulou, V. Psycharis, and C. D. Samara (2011). Dalton Trans. 40, 7946.
A. B. P. Lever Inorganic Electronic Spectroscopy (Elsevier, Amsterdam, 1984).
J. S. Kwag, M. H. Jeong, A. J. Laugh, and J. C. Kim (2010). Bull. Korean Chem. Soc. 31, 2069.
Z. A. Siddiqi and V. J. Mathew (1994). Polyhedron 13, 799.
I. A. Ansari, F. Sama, M. Shahid, Rahisuddin, R. Arif, M. Khalid, and Z. A. Siddiqi (2016). RSC Adv. 6, 11088 (Add JCC).
C. D. Samara, P. D. Janakoudakis, D. P. Kessissaglou, G. E. Manoyussakis, D. Mentzapfos, and A. Terzis (1992). Dalton Trans. 3259.
B. N. Figgis and J. Lewis in J. Lewis and R. G. Wilkins (eds.), Modern Coordination Chemistry (Interscience Publishers Inc., New York, 1960), p. 400.
R. J. Angelici, Synthesis and Technique in Inorganic Chemistry, 2nd ed. (W.B. Saunders Company, Philadelphia, 1977), p. 198.
S. A. Chavan, D. Srinivas, and P. Ratnasamy (2001). Chem. Commun. 1124.
M. Łukasiewicz, Z. Ciunik, J. Mazurek, J. Sobczak, A. Staroń, S. Wołowiec, and J. J. Ziółkowski (2001). Eur. J. Inorg. Chem. 1575.
Acknowledgements
The authors are thankful to the Chairman, Department of Chemistry, Aligarh Muslim University, Aligarh, India, for providing necessary facilities. Farasha Sama thanks UGC, New Delhi for Senior Research Fellowship. M. Shahid acknowledges SERB-DST, New Delhi for financial assistance (Ref. No. SR/FT/CS-76/2011) and Z. A. Siddiqi thanks UGC, New Delhi for UGC BSR faculty fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sama, F., Ansari, I.A., Raizada, M. et al. Design, Structural Characterization and Catalytic Activity of Incomplete Dicubane Clusters of N-Substituted Diethanolamines. J Clust Sci 28, 1355–1377 (2017). https://doi.org/10.1007/s10876-016-1145-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1145-1