Abstract
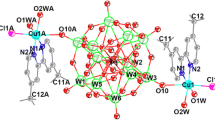
Two proton-conductive complexes based-on decorated Keggin-type clusters, {[Cu(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (1) and {[Zn(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (2) (where dmphen is 4,7-dimethyl-1,10-phenanthroline and DMF is N,N-dimethylformamide), were simply synthesized by the reaction of H4SiW12O40·24H2O, CuCl2·6H2O/ZnCl2 and dmphen at room temperature. The products were structurally characterized by elemental analyses, infrared spectroscopy, and single-crystal X-ray diffraction analyses. Single-crystal X-ray diffraction analyses at 293 K revealed that two complexes both crystallized in the monoclinic space group C2/c and presented two three-dimensional supramolecular networks with one-dimensional hydrophilic channels constructed by decorated Keggin-type clusters and solvent water molecules via the hydrogen-bonding interactions. The results of thermogravimetric analyses suggest that two complexes have good water holding in one-dimensional hydrophilic channels in the temperature range 20–100 °C. Two Complexes exhibit good proton conductivities (over 10−5 S cm−1 for 1 and over 10−4 S cm−1 for 2) at 100 °C in the relative humidity range 35–98%.






Similar content being viewed by others
References
H. Mansouri-Torshizi, M. Saeidifar, A. Divsalar, and A. A. Saboury (2010). Spectrochim. Acta A 77, 312.
G. Faraglia, S. Sitran, and D. Montagner (2005). Inorg. Chim. Acta 258, 971.
C. A. Johns, G. M. Golzar-Hossain, K. M. Abdul-Malik, S. Zahir-Haider, and U. K. Rowzatur-Romman (2001). Polyhedron 20, 721.
M. J. Plater, M. R. Foreman, M. S. Skakle, and R. A. Howie (2002). Inorg. Chim. Acta 332, 135.
A. M. Thumas, A. D. Naik, M. Nethaji, and A. B. Chakravarty (2004). Inorg. Chim. Acta 357, 2315.
R. E. Shepherd, Y. Chen, R. A. Kortes, and M. S. Ward (2000). Inorg. Chim. Acta 303, 30.
Y. Wang and N. Okabe (2005). Inorg. Chim. Acta 358, 3407.
V. Amani, N. Safari, B. Notash, and H. R. Khavasi (2009). J. Coord. Chem. 62, 1939.
R. D. Willett, G. Pon, and C. Nagy (2001). Inorg. Chem. 40, 4342.
Q. X. Han, C. He, M. Zhao, B. Qi, J. Y. Niu, and C. Y. Duan (2013). J. Am. Chem. Soc. 135, 10186.
M. L. Wei, X. X. Wang, and X. Y. Duan (2013). Chem. Eur. J. 19, 1607.
C. M. Roch, E. Ayrault, L. Lisnard, J. Marrot, F. X. Liu, and F. Secheresse (2006). J. Clust. Sci. 17, 283.
Y. W. Liu, S. M. Liu, X. Y. Lai, J. Miao, D. F. He, N. Li, F. Luo, Z. Shi, and S. X. Liu (2015). Adv. Funct. Mater. 25, 4480.
E. L. Zhou, C. Qin, P. Huang, X. L. Wang, W. C. Chen, K. Z. Shao, and Z. M. Su (2015). Chem. Eur. J. 21, 11894.
D. Y. Du, J. S. Qin, S. L. Li, Z. M. Su, and Y. Q. Lan (2014). Chem. Soc. Rev. 43, 4615.
E. L. Zhou, C. Qin, X. L. Wang, K. Z. Shao, and Z. M. Su (2015). Chem. Eur. J. 21, 13058.
T. Dong, J. B. Du, M. Cao, and C. W. Hu (2010). J. Clust. Sci. 21, 155.
M. L. Wei, H. H. Li, and X. X. Wang (2012). J. Clust. Sci. 23, 325.
C. Y. Duan, M. L. Wei, D. Guo, C. He, and Q. J. Meng (2010). J. Am. Chem. Soc. 132, 3321.
X. Wang, C. Y. Kong, J. J. Lai, and M. L. Wei (2016). J. Clust. Sci. 27, 645.
X. Wang, C. Y. Duan, C. Y. Kong, and M. L. Wei (2016). J. Coord. Chem. 69, 779.
C. R. De Silva, J. R. Maeyer, R. Wang, G. S. Nichol, and Z. Zheng (2007). Inorg. Chim. Acta 360, 3534.
I. Warad, M. Al-Ali, B. Hammouti, T. B. Hadda, R. Shareiah, and M. Rzaigui (2013). Res. Chem. Intermed. 39, 2451.
V. Singh, D. Singh, R. Kumar, S. Lata, and M. Sharma (2010). Asian J. Chem. 22, 5482.
SMART and SAINT Area Detector Control and Integration Software (Siemens Analytical X-ray Systems Inc, Madison, 1996).
G. M. Sheldrick SHELXTL Version 5.1, Software Reference Manual (Bruker AXS Inc, Madison, 1997).
I. D. Brown and D. Altermatt (1985). Acta Crystallogr. Sect. B Struct. Sci. 41, 244.
M. L. Wei, J. J. Sun, and X. Y. Duan (2014). Eur. J. Inorg. Chem. 2014, 345.
S. C. Nyburg and C. H. Faerman (1985). Acta Crystallogr. Sect. B Struct. Sci. 41, 274.
H. Strasdeit, I. Büsching, S. Behrends, W. Saak, and W. Barklage (2001). Chem. Eur. J. 7, 1133.
O. Nakamura, T. Kodama, I. Ogino, and Y. Miyake (1979). Chem. Lett. 1, 17.
K. D. Kreuer, A. Rabenau, and W. Weppner (1982). Angew. Chem. Int. Ed. 21, 208.
R. D. Hancock and A. E. Martell (1989). Chem. Rev. 89, 1875.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21171050 and 21501047).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kong, CY., Duan, XY., Lai, JJ. et al. Syntheses, Structures and Proton Conductivities of Two Complexes Based on Decorated Keggin-Type Clusters: {[M(dmphen)(DMF)2(H2O)]2[SiW12O40]}·6H2O (M = Cu and Zn; dmphen = 4,7-dimethyl-1,10-phenanthroline). J Clust Sci 28, 1407–1420 (2017). https://doi.org/10.1007/s10876-016-1144-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1144-2