Abstract
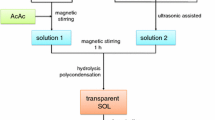
Yttria-stabilized zirconia (YSZ) nano-clusters were synthesized by a sol–gel process. The aim was to produce YSZ powders in order to prepare thick coatings for thermal barrier to be applied on gas turbine engine components. Yttrium nitrate hexahydrate and zirconium oxy-chloride octahydrate were used as a source of zirconium, citric acid was taken as a chelating agent, and ethylene glycol was used as a polysterification agent. The synthesized powders were characterized by X-ray diffraction, transmission electron microscopy, thermo-gravimetric analysis and differential scanning calorimetry, and Raman spectroscopy. Furthermore, parameters were critically analyzed in order to synthesize non-transformable (t′) tetragonal crystal structure, which is the best zirconia phase for high temperature thermal barrier coatings applications. In this regard, tetragonal YSZ nano-clusters were heated in an alumina crucible at a temperature of 1200 °C for 100 h.






Similar content being viewed by others
References
L. Kumari, W. Li, J. Xu, R. Leblanc, D. Wang, Y. Li, H. Guo, and J. Zhang (2009). Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 9, (9), 3874–3880.
I. Freris, P. Riello, F. Enrichi, D. Cristofori, and A. Benedetti (2011). Opt. Mater. 33, 1745–1752. doi:10.1016/j.optmat.2011.06.010.
J. He, J. Chen, L. Ren, Y. Wang, C. Teng, M. Hong, et al. (2014). ACS Appl. Mater. Interfaces 6, 2718–2725. doi:10.1021/am405202d.
H. Uchiyama, K. Takagi, and H. Kozuka (2012). Colloids Surf. A Physicochem. Eng. Asp. 403, 121–128. doi:10.1016/j.colsurfa.2012.03.065.
D. R. Clarke and C. G. Levi (2003). Annu. Rev. Mater. Res. 33, 383–417. doi:10.1146/annurev.matsci.33.011403.113718.
T. Koch and P. Ziemann (1996). Appl. Surf. Sci. 99, 51–57. doi:10.1016/0169-4332(95)00512-9.
J. H. Shim, C. Chao, H. Huang, and F. B. Prinz (2007). Chem. Mater. 19, 3850–3854. doi:10.1021/cm070913t.
T. Miller and V. Grassian (1995). J. Am. Chem. Soc. 117, 10969–10975. doi:10.1021/ja00149a020.
Y. Li, D. He, Z. Cheng, C. Su, J. Li, and Q. Zhu (2001). J. Mol. Catal. A Chem. 175, 267–275. doi:10.1016/S1381-1169(01)00233-3.
D. Chen, L. Cao, F. Huang, P. Imperia, Y.-B. Cheng, and R. A. Caruso (2010). J. Am. Chem. Soc. 132, 4438–4444. doi:10.1021/ja100040p.
D. Lu, J. Wang, L. Wang, D. Du, C. Timchalk, R. Barry, et al. (2011). Adv. Funct. Mater. 21, 4371–4378. doi:10.1002/adfm.201100616.
M. Zhou and A. Ahmad (2006). Mater. Res. Bull. 41, 690–696. doi:10.1016/j.materresbull.2005.10.018.
A. Subramanian, P. W. Carr, and C. V. McNeff (2000). J. Chromatogr. A 890, 15–23. doi:10.1016/S0021-9673(00)00289-2.
B. Yan, C. McNeff, and F. Chen (2001). J. Am. Ceram. Soc. 27, 1721–1727. doi:10.1111/j.1151-2916.2001.tb00905.x.
B. Yan, C. V. McNeff, P. W. Carr, and A. V. McCormick (2005). J. Am. Ceram. Soc. 88, 707–713. doi:10.1111/j.1551-2916.2005.00133.x.
A. Pattanayak and A. Subramanian (2009). Powder Technol. 192, 359–366. doi:10.1016/j.powtec.2009.01.023.
A. Pattanayak and A. Subramanian (2011). Int. J. Appl. Ceram. Technol. 8, 94–111. doi:10.1111/j.1744-7402.2009.02410.x.
H. Liu, H. Jazi, M. Bussmann, and J. Mostaghimi (2009). Experiments and modeling of rapid solidification of plasma-sprayed yttria-stabilized zirconia. Acta Mater. 57, (20), 6013–6021.
C. Viazzi, J.-P. Bonino, F. Ansart, and A. Barnabé (2008). Structural study of metastable tetragonal YSZ powders produced via a sol–gel route. J. Alloys Compd. 452, (2), 377–383.
G. Stefanic, S. Music, B. Grzeta, S. Popovic, and A. Sekulic (1998). Influence of pH on the stability of low temperature t-ZrO2. J. Phys. Chem. Solids 59, (6), 879–885.
G. Pacheco and J. Fripiat (2000). Physical chemistry of the thermal transformation of mesoporous and microporous zirconia. J. Phys. Chem. B 104, (50), 11906–11911.
I. Nettleship and R. Stevens (1987). Tetragonal zirconia polycrystal (TZP)—a review. Int. J. High Technol. Ceram. 3, (1), 1–32.
E. Subbarao (1981). Ceramic dielectrics for capacitors. Ferro-electrics 35, (1), 143–148.
H. Scott (1975). Phase relationships in the zirconia-yttria system. J. Mater. Sci. 10, (9), 1527–1535.
R. Mevrel, C. Rio, M. Poulain, C. Diot, and F. Nardou (1987). Technical Report No. 28/2019M. ONERA.
R. A. Miller, J. L. Smialek, and R. G. Garlick (1981). Phase stability in plasma-sprayed, partially stabilized zirconia-yttria. J. Am. Ceram. Soc. 3, 241.
R. A. Miller (1997). Thermal barrier coatings for aircraft engines: history and directions. J. Therm. Spray. Technol. 6, (1), 35–42.
S. Bose High temperature coatings (Butterworth-Heinemann, UK, 2011).
C. Viazzi, F. Ansart, and J. P. Bonino Proceeding of the Poudres et Materiaux ´Frittes 2005 (Cherbourg, France, 2005).
M. Pechini (1967). Patent No. 3,330,697. United States Patent Office.
B. D. Cullity and S. R. Stock Elements of X-ray diffraction, vol. 3 (Prentice Hall Upper Saddle River, NJ, 2001).
R. S. da Silva, M. I. B. Bernardi, and A. C. Hernandes (2007). Synthesis of non-agglomerated Ba0.77Ca0.23TiO3 nanopowders by a modified polymeric precursormethod. J. Sol-Gel Sci. Technol. 42, (2), 173–179.
K. Singh, L. Pathak, and S. Roy (2007). Effect of citric acid on the synthesis of nano-crystalline yttria stabilized zirconia powders by nitrate–citrate process. Ceram. Int. 33, (8), 1463–1468.
S. Sakka Handbook of sol-gel science and technology. 1. Sol-gel processing, vol. 1 (Springer, New York, 2005).
M. Kakihana and M. Yoshimura (1999). Synthesis and characteristics of complex multicomponent oxides prepared by polymer complex method. Bull. Chem. Soc. Jpn. 72, (7), 1427–1443.
Y. Xu, X. Yuan, G. Huang, and H. Long (2005). Polymeric precursor synthesis of Ba2Ti9O20. Mater. Chem. Phys. 90, (2), 333–338.
G. Socrates and G. Socrates Infrared and Raman characteristic group frequencies: tables and charts (Wiley, Chichester, 2001).
Y. Zhang, A. Li, Z. Yan, G. Xu, C. Liao, and C. Yan (2003). (ZrO2)0.85 (REO1.5)0.15. Res. J. Solid State Chem. 171, (1–2), 434–438.
C. Laberty-Robert, F. Ansart, C. Deloget, M. Gaudon, and A. Rousset (2001). Powder synthesis of nanocrystalline ZrO2—8 % Y2O3 via a polymerization route. Mater. Res. Bull. 36, (12), 2083–2101.
Y.-W. Zhang, Z.-G. Yan, F.-H. Liao, C.-S. Liao, and C.-H. Yan (2004). Citrate gel synthesis and characterization of (ZrO2)0.85 (REO1.5)0.15 (RE = Y, Sc) solid solutions. Mater. Res. Bull. 39, (11), 1763–1777.
D. Thackeray (1974). The Raman spectrum of zirconium dioxide. Spectrochimica Acta A Mol. Spectrosc. 30, (2), 549–550.
C. Li and M. Li (2002). UV Raman spectroscopic study on the phase transformation of ZrO2, Y2O3–ZrO2 and SO42−/ZrO2. J. Raman Spectrosc. 33, (5), 301–308.
A. Naumenko, N. Berezovska, M. Biliy, and O. Shevchenko (2008). Vibrational analysis and Raman spectra of tetragonal zirconia. Phys. Chem. Solid State 9, (1), 121–125.
D. Gazzoli, G. Mattei, and M. Valigi (2007). Raman and X-ray investigations of the incorporation of Ca2+ and Cd2+ in the ZrO2 structure. J. Raman Spectrosc. 38, (7), 824–831.
C. Perry, D. W. Liu, and R. P. Ingel (1985). Phase characterization of partially stabilized zirconia by Raman spectroscopy. J. Am. Ceram. Soc. 68, (8), C184–C187.
D. J. Kim, H. J. Jung, and I. S. Yang (1993). Raman spectroscopy of tetragonal zirconia solid solutions. J. Am. Ceram. Soc. 76, (8), 2106–2108.
Acknowledgments
The authors gratefully acknowledge the financial support of the Ministry of Education and Science of the Russian Federation in the framework of Increase Competitiveness Program of NUST «MISiS» (Grant No. К4-2014-081) and experimental support of National University of Science and Technology “MISiS”, Moscow, Russia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tailor, S., Singh, M. & Doub, A.V. Synthesis and Characterization of Yttria-Stabilized Zirconia (YSZ) Nano-Clusters for Thermal Barrier Coatings (TBCs) Applications. J Clust Sci 27, 1097–1107 (2016). https://doi.org/10.1007/s10876-016-1014-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1014-y