Abstract
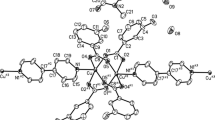
In this paper, self-assembly reactions of copper(II) ions, methoxybenzoate isomers and 2,2′-bipyridine yield two copper-oxygen polynuclear complexes: [Cu2(bpy)2(2-C8H7O3)3]·(2-C8H7O3)·14H2O 1, [Cu4(bpy)4(H2O)(OH)4]·4(3-C8H7O3)·17H2O 2, and a simple mononuclear complex [Cu(bpy)(H2O)(4-C8H7O3)2] 3. (bpy = 2,2′-bipyridine, C8H7O3 = methoxybenzoate ion). Single crystal X-ray diffraction analyses reval that compound 1 is a dinuclear copper(II) complex which bridged by three carboxylate groups, and 2 presents a discrete step-like tetra-nuclear copper Cu4O4 core. Compound 3 shows a square pyramidal mononuclear geometry. The magnetic susceptibility of complex 1 measured from 2 to 300 K, revealed an antiferromagnetic interaction between the Cu(II) ions. Furthermore, the results about IR spectra and thermal analyses were discussed.








Similar content being viewed by others
References
O. Kahn Molecular Magnetism (VCH, New York, 1993).
E. I. Solomon, P. Chen, M. Metz, S. K. Lee, and A. E. Palmer (2001). Angew. Chem. Int. Ed. 40, 4570–4590.
J. Fielden, J. Sprott, D. L. Long, P. Kogerler, and L. Cronin (2006). Inorg. Chem. 45, 2886–2895.
M. Albrecht (2001). Chem. Rev. 101, 3457–3497.
S. Y. Zhang, X. P. Zhang, H. M. Li, Z. Niu, W. Shi, and P. Cheng (2015). Inorg. Chem. 54, 2310–2314.
K. Zhao, Y. Jiang, X. Qiu, J. Tian, and X. Li (2011). J. Coord. Chem. 64, 1375–1384.
J. Yin, T. T. Yin, C. Gao, B. W. Wang, Y. Y. Zhu, Z. Q. Wu, and S. Gao (2014). Eur. J. Inorg. Chem. 31, 5385–5390.
K. Das, T. K. Mondal, E. Garribba, M. Fondo, C. Sinha, and A. Datta (2014). Inorg. Chim. Acta 413, 194–202.
A. D. Burrows, K. Cassar, M. F. Mahon, and J. E. Warren (2007). Dalton Trans. 24, 2499–2509.
P. de Meester and A. C. Skapski (1972). J. Chem. Soc. Dalton Trans. 22, 2400–2404.
J. M. Espstein, B. N. Figgis, A. H. White, and A. C. Willis (1974). J. Chem. Soc. Dalton Trans. 18, 1954–1961.
L. M. Mirica and T. D. P. Stack (2005). Inorg. Chem. 44, 2131–2133.
S. Knoner, S. Saha, K. I. Okamoto, and J. P. Tuchagues (2003). Inorg. Chem. 42, 4668–4672.
R. Acevedo-Chávez, M. E. Costas, S. Bernès, G. Medina, and L. Gasque (2002). J. Chem. Soc. Dalton Trans. 12, 2553–2558.
R. W. Saalfrank, C. Schmidt, H. Maid, F. Hampel, W. Bauer, and A. Scheurer (2006). Angew. Chem. Int. Ed. 45, 315–318.
B. Abarca, R. Ballesteros, M. Chadlaoui, C. R. de Arellano, and J. A. Real (2007). Eur. J. Inorg. Chem. 29, 4574–4578.
X. Li, D. Y. Cheng, J. L. Lin, Z. F. Li, and Y. Q. Zheng (2008). Cryst. Growth Des. 8, (8), 2853–2861.
Y. Q. Zheng, D. Y. Cheng, B. B. Liu, and W. X. Huang (2011). Dalton Trans. 40, 277–286.
G. M. Sheldrick SHELXL-97, Program for Crystal Structure Refinement (University of Göttingen, Göttingen, 1997).
G. M. Sheldrick SHELXS-97, Program for Crystal Structure Solution (University of Göttingen, Göttingen, 1997).
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijin, and G. C. Verschoor (1984). J. Chem. Soc. Dalton Trans. 7, 1349.
B. Graham, M. T. W. Hearn, P. C. Junk, C. M. Kepert, F. E. Mabbs, B. Moubaraki, K. S. Murray, and L. Spiccia (2001). Inorg. Chem. 40, 1536–1543.
G. Alzuet, J. A. Real, J. Borrás, R. Santiago-García, and S. García-Granda (2001). Inorg. Chem. 40, 2420–2423.
K. NaKamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed (Wiley, New York, 1986).
O. Kahn Molecular Magnetism (VCH, Weinheim, 1993), pp. 135–141.
Acknowledgments
This project was supported by the Department of Education of Zhejiang Province and K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, JQ., Zheng, YQ., Zhu, HL. et al. Synthesis, Crystal Structures and Magnetic Properties of Three New Polynuclear Cu(II) Complexes Based on the Methoxybenzoate Isomers. J Clust Sci 27, 485–499 (2016). https://doi.org/10.1007/s10876-015-0941-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0941-3