Abstract
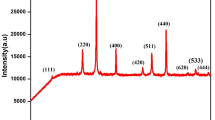
Gadolinium substituted cobalt ferrite nanocrystals with composition of CoFe2−xGdxO4 (x = 0–0.04 in a step of 0.01) were prepared by a hydrothermal process and without subsequent annealing. X-ray diffraction, field-emission scanning electron microscopy, and vibrating sample magnetometer were used to investigate the effect of Gd3+ cation substitution on structural Characteristics and magnetic properties of cobalt ferrite nanocrystals. The X-ray diffraction analysis demonstrated that single phase spinel ferrites were obtained. The FE-SEM micrographs of the synthesized samples indicated the presence of two distinct groups of grains exhibiting different sizes and, more important, different shapes. The results of magnetic hysteresis at a room temperature showed that with an increase in gadolinium content, the coercive field decreased from 1250 Oe for x = 0 to 450 Oe for x = 0.03. In addition, it was observed that with substitutions of gadolinium cations, the values of saturation magnetization decreased.






Similar content being viewed by others
References
S. Chang and Q. Haoxue (2009). IEEE Trans. Magn. 34, 1111.
M. E. Mata-Zamora, H. Montiel, G. Alvarez, J. M. Saniger, R. Zamorano, and R. Valenzuele (2007). J. Magn. Magn. Mater. 316, e532.
O. Caltun, I. Dumitru, M. Feder, N. Lupu, and H. Chiriac (2008). J. Magn. Magn. Mater. 320, e869.
D. H. Kim, D. E. Nikles, D. T. Johnson, and C. S. Brazel (2008). J. Magn. Magn. Mater. 320, 2390.
L. Kumar and M. Kar (2012). Ceram. Int. 38, 4771.
A. M. Pachpinde, M. M. Langade, K. S. Lohar, S. M. Patange, and S. Shirsath (2014). Chem. Phys. 429, 20.
A. K. Nikumbh, R. A. Pawar, D. V. Nighot, G. S. Gugale, M. D. Sangale, M. B. Khanvilkar, and A. V. Nagawade (2014). J. Magn. Magn. Mater. 355, 201.
M. M. Ruiz, J. L. Mietta, P. S. Antonel, O. E. Perez, R. M. Negri, and G. Jorge (2013). J. Magn. Magn. Mater. 327, 11.
X. Zhao, W. Wang, Y. Zhang, S. Wu, F. Li, and J. P. Liu (2014). Chem. Eng. J. 250, 164.
S. Urcia Romero, O. Perales Perez, and G. Gutierrez (2010). J. Appl. Phys. 107, 09A508.
T. Sodaee, A. Ghasemi, E. Paimozd, A. Paesano Jr, and A. Morisako (2013). J. Magn. Magn. Mater. 330, 169.
T. Sodaee, A. Ghasemi, E. Paimozd, A. Paesano Jr, and A. Morisako (2013). J. Electron. Mater. 42, 2771.
J. Peng, M. Hojamberdiev, Y. Xu, B. Cao, J. Wang, and H. Wu (2011). J. Magn. Magn. Mater. 323, 133.
S. Amiri and H. Shokrollahi (2013). J. Magn. Magn. Mater. 345, 18.
A. Rafferty, T. Prescott, and D. Brabazon (2008). Ceram. Int. 34, 15.
M. M. El-Okr, M. A. Salem, M. S. Salim, R. M. El-Okr, M. Ashoush, and H. M. Talaat (2011). J. Magn. Magn. Mater. 323, 920.
M. T. Rahman and C. V. Ramana (2014). Ceram. Int. 40, (9), 14533.
E. Pervaiz and I. H. Gul (2013). J. Phys. Conf. Ser. 439, 1.
W. C. Kim, S. J. Kim, and C. S. Kim (2002). J. Appl. Phys. 91, 7607.
Md T Rahman, M. Vargas, and C. V. Ramana (2014). J. Alloy. Compd. 617, 547.
S. C. Goh, C. H. Chia, S. Zakaria, M. Yusoff, C. Y. Haw, S. Ahmadi, N. M. Huang, and H. N. Lim (2010). Mater. Chem. Phys. 120, 31.
X. Liu, S. Y. Fu, and L. P. Zhou (2007). J. Solid State Chem. 180, 461.
D. S. Mathew and R. S. Juang (2007). Chem. Eng. J. 129, 51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sodaee, T., Ghasemi, A. & Razavi, R.S. Microstructural Characteristics and Magnetic Properties of Gadolinium-Substituted Cobalt Ferrite Nanocrystals Synthesized by Hydrothermal Processing. J Clust Sci 27, 1239–1251 (2016). https://doi.org/10.1007/s10876-015-0925-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0925-3