Abstract
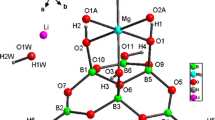
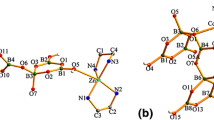
Two new tetraborates, namely [Li(H2O)4]2[Cu(H2O)6][Cu(H2O)5]2[B4O5(OH)4]4·4H2O (1) and [Cu(en)2(H2O)2][Cu(en)2(B(OH)3)2][B4O5(OH)4]2·H2O (2) have been made under solvothermal conditions and characterized by FT-IR spectroscopy, powder X-ray diffraction, single crystal X-ray diffraction and thermogravimetric analysis, respectively. 1: triclinic, Pī, a = 7.0400(2) Å, b = 7.8779(3) Å, c = 25.8958(11) Å, α = 90.827(3)º, β = 95.985(3)º, γ = 105.816(3)º, Z = 1. 2: monoclinic, P2/c, a = 12.5723(6) Å, b = 9.1508(5) Å, c = 15.9800(6) Å, β = 94.742(4)º, Z = 2. In structure 1, the B4O5(OH)4 clusters join together via H-bonding interactions to produce 3-D supramolecular framework with three types of channels located by the Cu(H2O)6, Cu(H2O)5 and Li(H2O)4 polyhedra, respectively. While in 2, the B4O5(OH)4 clusters link each other to form 2-D layers and then further pillared by Cu(en)2(B(OH)3)2 complexes via H-bonding interactions, resulting in 3-D supramolecular framework with hexagonal and rectangle channels, in which the rectangle channels are filled by Cu(H2O)2(en)2 complexes.





Similar content being viewed by others
References
J. D. Grice, P. C. Burns, and F. C. Hawthorne (1999). Can. Mineral. 37, 731.
M. S. Wang, G. C. Guo, W. T. Chen, G. Xu, W. W. Zhou, K. J. Wu, and J. S. Huang (2007). Angew. Chem. Int. Ed. 46, 3909.
S. Wang, N. Ye, W. Li, and D. Zhao (2010). J. Am. Chem. Soc. 132, 8779.
C. T. Chen, Y. B. Wang, B. C. Wu, K. C. Wu, W. L. Zeng, and L. H. Yu (1995). Nature. 373, 322.
H. Wu, S. Pan, K. R. Poeppelmeier, H. Li, D. Jia, Z. Chen, X. Fan, Y. Yang, J. M. Rondinelli, and H. Luo (2011). J. Am. Chem. Soc. 133, 7786.
P. C. Burns (1995). Can. Miner. 33, 1167.
H. Wu, H. Yu, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier, and J. M. Rondinelli (2013). J. Am. Chem. Soc. 135, 4215.
Z. Y. Wu, P. Brandao, and Z. Lin (2012). Inorg. Chem. 51, 3088.
C. T. Chen, S. Y. Luo, X. Y. Wang, G. L. Wang, X. H. Wen, H. X. Wu, X. Zhang, and Z. Y. Xu (2009). J. Opt. Soc. Am. B. 26, 1519.
C. Heyward, C. McMillen, and J. Kolis (2012). Inorg. Chem. 51, 3956.
M. Touboul, N. Penin, and G. Nowogrocki (2003). Solid State Sci. 5, 1327.
G. Aka and A. Brenier (2003). Optical Materials 22, 89–94.
C. Chen, B. Wu, A. Jiang, G. You (1985). Sci. Sin. Ser. B (Engl. Ed.) 28, 235.
C. T. Chen, Y. C. Wu, A. D. Jiang, G. M. You, R. K. Li, and S. J. Lin (1989). J. Opt. Soc. Am. B. 6, 616.
Y. Mori, I. Kuroda, S. Nakajima, T. Sasaki, and S. Nakai (1995). Appl. Phys. Lett. 67, 1818.
Y. C. Wu, T. Sasaki, A. Yokotani, H. Tang, and C. T. Chen (1993). Appl. Phys. Lett. 62, 2614.
A. Borsutzky, R. Brünger, C. Huang, and R. Wallenstein (1991). Appl. Phys. 52, 55.
G. M. Wang, Y. Q. Sun, and G. Y. Yang (2004). J. Solid State Chem. 177, 4648.
Z. E. Lin and G. Y. Yang (2011). Eur. J. Inorg. Chem. 26, 3857.
C. Rong, Z. W. Yu, Q. Wang, S. T. Zheng, C. Y. Pan, F. Deng, and G. Y. Yang (2009). Inorg. Chem. 48, 3650.
J. Zhou, W. H. Fang, C. Rong, and G. Y. Yang (2010). Chem. Eur. J. 16, 4852.
G. M. Wang, J. H. Li, H. L. Huang, H. Li, and J. Zhang (2008). Inorg. Chem. 47, 5039.
L. Z. Wu, L. Cheng, J. N. Shen, and G. Y. Yang (2013). CrystEngComm. 15, 4483.
M. C. Liu, P. Zhou, H. G. Yao, S. H. Ji, R. C. Zhang, M. Ji, and Y. L. An (2009). Eur. J. Inorg. Chem. 31, 4622.
A. K. Paul and S. Natarajan (2010). Crystal Growth & Design. 10, 765.
Z. H. Liu, L. Q. Li, and W. J. Zhang (2006). Inorg. Chem. 45, 1430.
K. Byrappa and M. Yoshimura Handbook of Hydrothermal Technology, Chapter 2: History of Hydrothermal Technology (Noyes publications, New york, 2001).
M. Li, J. Z. Chang, Z. L. Wang, and H. Z. Shi (2006). J. Solid State Chem. 179, 3265.
S. H. Yang, G. H. Li, S. J. Tian, F. H. Liao, and J. H. Lin (2007). Crystal Growth & Design. 7, 1246.
H. Y. Sung, M. M. Wu, and I. D. Williams (2000). Inorg. Chem. Comm. 3, 401.
Y. Yang, Y. Wang, J. Zhu, R. B. Liu, J. Xu, and C. G. Meng (2011). Z. Anorg. Allg. Chem. 637, 735.
Y. Yang, Y. Wang, J. Sun, M. Cui, and C. G. Meng (2011). Z. Anorg. Allg. Chem. 637, 729.
R. Janda, G. Heller, and J. Z. Pickardt (1981). Kristall 154, 1.
L. X. Zhu, T. Yue, and S. Y. Gao (2003). J. Mol. Struct. 658, 215.
J. G. Zhou, F. Y. Zhao, and Q. Yang (2006). Thermochim Acta. 448, 52.
M. Touboul, N. Penin, and G. Nowogrocki (1999). J. Solid State Chem. 143, 260.
G. M. Sheldrick, A program for the Siemens Area Detector ABSorption correction, University of Göttingen, 1997.
G. M. Sheldrick SHELXS-97 Program for Solution of Crystal Structures (University of Göttingen, Göttingen, 1997).
C. L. Christ and J. R. Clark (1977). Phys. Chem. Miner. 2, 59.
C. Hormillosa, S. Healy, T. Stephen, I. D. Brown, Bond Valence Calculator, Version 2.0, 1993, Original at http://ccp14.sims.nrc.ca/ccp/web-mirrors/i_d_brown/.
J. Krogh-Moe (1965). Phys. Chem. Glasses. 6, 46.
Acknowledgments
This work was supported by the NSFC (nos. 91122028, 50872133 and 21201017), the NSFC of Distinguished Young Scholars (No. 20725101), and the 973 Program (Nos. 2014CB932101).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, G., Pan, R., He, H. et al. Two Borate Supramolecular Frameworks Based on B4O5(OH)4 Cluster Units. J Clust Sci 26, 1993–2003 (2015). https://doi.org/10.1007/s10876-015-0894-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-015-0894-6