Abstract
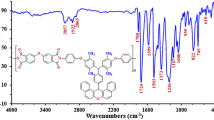
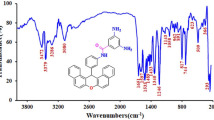
New poly(ether-amide) nanocomposite containing phosphine oxide was prepared via solution polymerization process from synthesized poly(ether-amide) and Fe3O4 nanoparticles in a solution of N,N-dimethylformamide. Uniform monodisperse Fe3O4 nanoparticles were synthesized at room temperature via a facile sonochemical reaction. Poly(ether-amide) (PEA) as the polymer matrix was synthesized from reaction of 1,4-(4-carboxy phenoxy)butane (1) and bis(3-amino phenyl)phenyl phosphine oxide (2) via a direct polycondensation reaction. Nanoparticle and nanocomposite were characterized using X-ray diffraction, scanning electron microscopy, transmission electron microscopy and Fourier transform infrared. The effect of the presence of Fe3O4 nanoparticles on the thermal properties of PEA was studied using thermogravimetric analysis in nitrogen atmospheres. The magnetic properties of the sample were also investigated using an alternating gradient force magnetometer. We found that the Fe3O4 nanoparticles exhibit a ferromagnetic behaviour with a saturation magnetization of 59 emu/g and a coercivity of 104 Oe at room temperature. The coercivity of PEA/Fe3O4 nanocomposites is found to be 126 Oe, higher than 104 Oe which is obtained for Fe3O4.











Similar content being viewed by others
References
V. Srivastava, P. K. Singh, C. H. Weng, and Y. C. Sharma (2011). Pol. J. Chem. Technol. 13, 1.
B. Y. Geng, J. Z. Ma, and J. H. Cryst (2008). Growth Des. 8, 5.
G. Nabiyouni and D. Ghanbari (2012). J. Appl. Polym. Sci. 125, 3268.
M. Yousefi, M. Salavati-Niasari, F. Gholamian, D. Ghanbari, and A. Aminifazl (2011). Inorg. Chim. Acta 371, 5.
B. Feng, R. Y. Hong, L. S. Wang, L. Guo, H. Z. Li, J. Ding, Y. Zheng, and D. G. Wei (2008). Colloids Surf. A 328, 52.
J. Qu, G. Liu, Y. Wang, and R. Hong (2010). Adv. Powder Technol. 21, 461.
G. Li, Y. Jiang, K. Huang, P. Ding, and J. Chen (2008). J. Alloy Compd. 466, 451.
J. Ren, M. Jia, T. Ren, W. Yuan, and Q. Tan (2008). Mater. Lett. 62, 4425.
G. Wua, X. Cai, X. Lin, and H. Yui (2010). React. Funct. Polym. 70, 732.
S. Qin, L. Wang, X. Zhang, and G. Su (2010). Appl. Surf. Sci. 257, 731.
F. Meng, R. Zhao, M. Xu, Y. Zhan, Y. Lei, J. Zhong, and X. Liu (2011). Colloids Surf. A 375, 245.
W. Zhang, S. Chen, W. Hu, B. Zhou, Z. Yang, N. Yin, and H. Wang (2011). Carbohydr. Polym. 86, 1760.
M. I. Sarwar, S. Zulfiqar, and Z. Ahmad (2008). Polym. Int. 57, 292.
D. A. Chatfield, I. N. Einhorn, R. W. Mickelson, and J. H. Futrell (1979). J. Polym. Sci. A Polym. Chem. 17, 1353.
M. Shabanian and D. Ghanbari (2013). J. Appl. Polym. Sci. 127, 2004.
S. Zulfiqar, Z. Ahmad, M. Ishaq, and M. I. Sarwar (2009). Mater. Sci. Eng. A 525, 30.
C. P. Yang, S. H. Hsiao, and C. C. Jang (1995). J. Polym. Sci. A Polym. Chem. 33, 1095.
C. P. Yang and J. H. Lin (1996). J. Polym. Sci. A Polym. Chem. 34, 341.
S. Zulfiqar, Z. Ahmad, and M. I. Sarwar (2007). Colloid Polym. Sci. 285, 1749.
K. Faghihi, M. Shabanian, M. Hajibeygi, and Y. Mohammadi (2010). J. Appl. Polym. Sci. 117, 1184.
K. Faghihi, M. Shabanian, and F. Shabani (2011). J. Polym. Res. 18, 637.
M. Hajibeygi and M. Shabanian (2012). J. Appl. Polym. Sci. 126, 280.
M. I. Sarwar, S. Zulfiqar, and Z. Ahmad (2007). J. Sol-Gel Sci. Technol. 44, 41.
K. Faghihi and M. Shabanian (2010). Macromol. Res. 18, 1148.
K. Faghihi and K. Zamani (2006). J. Appl. Polym. Sci. 101, 4263.
M. Shabanian, K. Faghihi, and F. Shabani (2012). Polym. Bull. 68, 375.
R. Jalajerdi, F. Gholamian, H. Shafie, A. Moraveji, and D. Ghanbari (2012). J. Nanostruct. 2, 105.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gholamian, F., Shabanian, M. & Shahrokh, M. Magnetic and Thermal Properties of Novel Poly(ether-amide)/Fe3O4 Nanocomposite Containing Phosphine Oxide Group. J Clust Sci 24, 177–188 (2013). https://doi.org/10.1007/s10876-012-0541-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-012-0541-4