Abstract
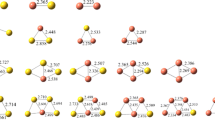
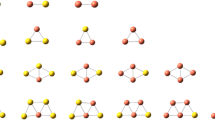
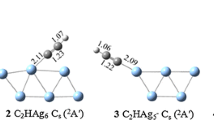
Density functional theory calculations were performed to investigate the structural and energetic properties of NO2 adsorption on small bimetallic Ag n Cu m clusters (n + m ≤ 5). Generally NO2 is adsorbed in bridge configuration. The adsorbates prefer Cu sites when both Ag and Cu co-exist in the clusters. The adsorption energies and the dissociation energies of the complex clusters increase as the Cu content increases for the given cluster size. Our calculation suggests that the bimetallic Ag n Cu m may react with NO2 dissociatively by way of Ag atom, Ag2 or AgCu loss. The N–O vibrational properties of the complex clusters were also discussed and analyzed.




Similar content being viewed by others
References
S. Sumiya, H. He, A. Abe, N. Takezawa, and K. Yoshida (1998). J. Chem. Soc. Faraday Trans. 94, 2217.
M. Valden, X. Lai, and D. W. Goodman (1998). Science 281, 1647.
F. Boccuzzi and A. Chiorino (2000). J. Phys. Chem. B 104, 5414.
A. M. Lamsabhi, M. Alcamí, O. Mó, M. Yáñez, J. Tortajada, and J. Y. Salpin (2007). ChemPhysChem 8, 181.
S. Sumiya, M. Saito, H. He, Q. C. Feng, N. Takezawa, and K. Yoshida (1998). Catal. Lett. 50, 87.
S. Kameoka, T. Chafik, Y. Ukisu, and T. Miyadera (1998). Catal. Lett. 55, 211.
F. Boccuzzi, S. Coluccia, G. Martra, and N. Ravasio (1999). J. Catal. 184, 316.
A. Citra, X. Wang, and L. Andrews (2002). J. Phys. Chem. A 106, 3287.
A. Sultana, M. Haneda, T. Fujitani, and H. Hamada (2007). Catal. Lett. 114, 96.
P. Sazama, L. Čapek, H. Drobná, Z. Sobalík, J. Dĕdeček, K. Arve, and B. Wichterlová (2005). J. Catal. 232, 302.
J. P. Breen, R. Burch, C. Hardacre, and C. J. Hill (2005). J. Phys. Chem. B 109, 4805.
S. Zhao, Y.-L. Ren, J. Wang, and W. P. Yin (2009). J. Phys. Chem. A 113, 1075.
H. Grönbeck, A. Hellman, and A. Gavrin (2007). J. Phys. Chem. A 111, 6062.
X. Ding, Z. Li, J. Yang, J. G. Hou, and Q. Zhu (2004). J. Chem. Phys. 121, 2558.
X. Li, A. E. Kuznetsov, H. F. Zhang, A. I. Boldyrev, and L. S. Wang (2001). Science 291, 859.
S. Shetty, S. Pal, and D. G. Kanhere (2003). J. Chem. Phys. 118, 7288.
V. E. Matulis and O. A. Ivaskevich (2006). Comput. Mater. Sci. 35, 268.
S. Chrétien, M. S. Gordon, and H. Metiu (2004). J. Chem. Phys. 121, 9931.
Q. Ge, C. Song, and L. Wang (2006). Comput. Mater. Sci. 35, 247.
C. Song, Q. Ge, and L. Wang (2005). J. Phys. Chem. B 109, 22341.
S. Zhao, Y. L. Ren, J. J. Wang, and W. P. Yin (2010). J. Phys. Chem. A 114, 4917.
A. M. Joshi, W. N. Delgass, and K. T. Thomson (2006). J. Phys. Chem. B 110, 23373.
M. M. Sadek and L. Wang (2006). J. Phys. Chem. A 110, 14306.
M. Neumaier, F. Weigend, O. Hampe, and M. M. Kappes (2008). Faraday Discuss. 138, 393.
D. Y. Wu, B. Ren, and Z. Q. Tian (2006). ChemPhysChem. 7, 619.
G. A. Bishea, N. Marak, and M. D. Morse (1991). J. Chem. Phys. 95, 5618.
D. A. Kilimis and D. G. Papageorgiou (2010). Eur. Phys. J. D 56, 189.
X. Lou, H. Gao, W. Wang, C. Xu, H. Zhang, and Z. Zhang (2010). J. Mol. Struct. (Theochem.) 959, 75.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al. Gaussian 03 (Gaussian, Inc., Pittsburgh, 2003).
J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh, and C. Fiolhais (1992). Phys. Rev. B 46, 6671.
P. J. Hay and W. R. Wadt (1985). J. Chem. Phys. 82, 270.
K. P. Huber and G. Herzberg Molecular Spectra and Molecular Structure: Constants of Diatomic Molecules (VanNostrand, New York, 1979).
S. Zhao, Z. H. Li, W. N. Wang, and K. N. Fan (2005). J. Chem. Phys. 122, 144701.
V. Bonačić-Koutecký, J. Burad, R. Mitrić, M. Ge, G. Zampella, and P. Fantucci (2002). J. Chem. Phys. 117, 3120.
V. Beutel, H. G. Krämer, G. L. Bhale, M. Kuhn, K. Weyers, and W. Demtröder (1993). J. Chem. Phys. 98, 2699.
R. A. Rohlfing and J. J. Valentini (1986). J. Chem. Phys. 84, 6560.
J. P. Foster and F. Weinhold (1980). J. Am. Chem. Soc. 102, 7211.
L. Cheng and Q. Ge (2008). J. Phys. Chem. C 112, 16924.
D. Mei, Q. Ge, J. Szanyi, and C. H. F. Peden (2009). J. Phys. Chem. C 113, 7779.
M. Neumaier, F. Weigend, O. Hampe, and M. M. Kappes (2006). J. Chem. Phys. 125, 104308.
Y. Zhao, Z. Li, and J. Yang (2009). Phys. Chem. Chem. Phys. 11, 2329.
S. Zhao, Y.-L. Ren, Y.-L. Ren, J. J. Wang, and W. P. Yin (2010). J. Mol. Struct. (Theochem.) 955, 66.
S. Zhao, Y.-L. Ren, Y.-L. Ren, J. J. Wang, and W. P. Yin (2011). Comput. Theor. Chem. 964, 298.
F. Viñes, A. Desikusumastuti, T. Staudt, A. Görling, J. Libuda, and K. N. Neyman (2008). J. Phys. Chem. C 112, 16539.
A. Fielicke, G. V. Helden, G. Meijer, B. Simard, and D. M. Rayner (2005). Phys. Chem. Chem. Phys. 7, 3906.
D. R. Lide, Handbook of Chemistry and Physics (CRC Press, Inc., Boca Raton, 1990–1991), 71st edn.
Acknowledgments
The project was supported by the Outstanding Talent Program of Henan Province (084200510015) and the Fund for Doctorates of Henan University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, S., Ren, Y., Lu, W. et al. Adsorption of NO2 on Small Silver Clusters with Copper Impurity: A Density Functional Study. J Clust Sci 23, 1039–1048 (2012). https://doi.org/10.1007/s10876-012-0493-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-012-0493-8