Abstract
Purpose
Home-based subcutaneous immunoglobulin (SCIg) therapy is an alternative to hospital-based intravenous infusions (IVIg). However, SCIg requires patient training and long-term support to ensure proper adherence, optimal efficacy and safety. We evaluated if switching patients to home-based SCIg including an interprofessional drug therapy management program (physician, community pharmacist and nurse) would be cost-effective within the Swiss healthcare system.
Methods
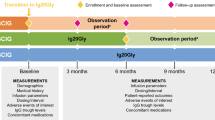
A 3-year cost-minimization analysis was performed from a societal perspective comparing monthly IVIg in an outpatient clinic and home-based weekly SCIg including an interprofessional program. Healthcare costs (immunoglobulin, professional time, infusion pump and disposables) were derived from administrative data. Transportation and productivity loss were estimated by expert opinion. The results were expressed in Swiss francs (CHF) and converted to Euros and US dollars (1 CHF = 0.92€, 1 CHF = $1.02; www.xe.com, 12/14/2015).
Results
Under base case assumptions, SCIg was estimated to cost 35,862 CHF (33,134€; $36,595) per patient during the first year and 30,309 CHF (28,004€; $30,929) in subsequent years versus 35,370 CHF (32,679€; $36,095) per year for IVIg. The total savings from switching to SCIg with the interprofessional program were 9630 CHF (8897€; $9828) per patient over 3 years. The results were relatively sensitive to the cost per gram of IgG, the cost of equipment and the annual number of infusions.
Conclusion
Home-based SCIg including an interprofessional therapy management program may be an efficient alternative for patients. The program provides long-term support from self-administration training to the responsible use of therapy (proper adherence, optimal efficacy and safety). Over the short term, additional costs from purchasing equipment and the drug therapy management program were offset by avoiding hospital costs.


Similar content being viewed by others
References
APIIEG. Consensus recommendations for the use of immunoglobulin replacement therapy in immune deficiency: Asia Pacific Immunoglobulins in Immunology Expert Group 2009.
Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142(1):1–11. doi:10.1111/j.1365-2249.2005.02834.x.
Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, et al. Global study of primary immunodeficiency diseases (PI)—diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011;51(1):61–70. doi:10.1007/s12026-011-8241-y.
Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9(6):722–8.
Wasserman RL. Progress in gammaglobulin therapy for immunodeficiency: from subcutaneous to intravenous infusions and back again. J Clin Immunol. 2012;32(6):1153–64. doi:10.1007/s10875-012-9740-x.
European Society for Immunodeficiencies (ESID). http://esid.org/. 2015.
Jolles S, Sleasman JW. Subcutaneous immunoglobulin replacement therapy with Hizentra, the first 20% SCIG preparation: a practical approach. Adv Ther. 2011;28(7):521–33. doi:10.1007/s12325-011-0036-y.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20(2):94–100.
Gardulf A, Nicolay U, Math D, Asensio O, Bernatowska E, Bock A, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42. doi:10.1016/j.jaci.2004.06.053.
Nicolay U, Kiessling P, Berger M, Gupta S, Yel L, Roifman CM, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26(1):65–72. doi:10.1007/s10875-006-8905-x.
Shapiro RS. Why I, use subcutaneous immunoglobulin (SCIG). J Clin Immunol. 2013;33 Suppl 2:S95–8. doi:10.1007/s10875-012-9853-2.
Simoens S. Pharmacoeconomics of immunoglobulins in primary immunodeficiency. Expert Rev Pharmacoecon Outcomes Res. 2009;9(4):375–86. doi:10.1586/erp.09.37.
Gardulf A, Andersen V, Bjorkander J, Ericson D, Froland SS, Gustafson R, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345(8946):365–9.
Hogy B, Keinecke HO, Borte M. Pharmacoeconomic evaluation of immunoglobulin treatment in patients with antibody deficiencies from the perspective of the German statutory health insurance. Eur J Health Econ. 2005;6(1):24–9. doi:10.1007/s10198-004-0250-5.
Beaute J, Levy P, Millet V, Debre M, Dudoit Y, Le Mignot L, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160(2):240–5. doi:10.1111/j.1365-2249.2009.04079.x.
Haddad L, Perrinet M, Parent D, Leroy-Cotteau A, Toguyeni E, Condette-Wojtasik G, et al. Economic evaluation of at home subcutaneous and intravenous immunoglobulin substitution. Rev Med Interne. 2006;27(12):924–6. doi:10.1016/j.revmed.2006.08.005.
Lazzaro C, Lopiano L, Cocito D. Subcutaneous vs intravenous administration of immunoglobulin in chronic inflammatory demyelinating polyneuropathy: an Italian cost-minimization analysis. Neurol Sci. 2014;35(7):1023–34. doi:10.1007/s10072-014-1632-9.
Cocito D, Serra G, Paolasso I, Barila DA, Lopiano L, Cattel L. Economic and quality of life evaluation of different modalities of immunoglobulin therapy in chronic dysimmune neuropathies. J Peripher Nerv Syst. 2012;17(4):426–8. doi:10.1111/j.1529-8027.2012.00444.x.
Liu Z, Albon E, GHyde C, WMHTA C. The effectiveness and cost effectiveness of immunoglobulin replacement therapy for primary immunodeficiency and chronic lymphocytic leukaemia: a systematic review and economic evaluation. University of Birmingham: Department of Public Health and Epidemiology2005.
Membe SK, Ho C, Cimon K, Morrison A, Kanani A, Roifman CM. Economic assessment of different modalities of immunoglobulin replacement therapy. Immunol Allergy Clin North Am. 2008;28(4):861–74. doi:10.1016/j.iac.2008.06.008.
Martin A, Lavoie L, Goetghebeur M, Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60. doi:10.1111/j.1365-3148.2012.01201.x.
Fasth A, Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28(4):370–8. doi:10.1007/s10875-008-9180-9.
Gardulf A, Moller G, Jonsson E. A comparison of the patient-borne costs of therapy with gamma globulin given at the hospital or at home. Int J Technol Assess Health Care. 1995;11(2):345–53.
Bourdin A, Berger J, Bugnon O. Immunoglobulin self-infusion: an interprofessional drug therapy management program. 42nd European Symposium on Clinical Pharmacy symposium on Clinical Pharmacy: Implementation of Pharmacy Practice; 16-18 October 2013; Prague, Czech Republic: International Journal of Clinical Pharmacy; 2013. p. 1281-2.
Bourdin A, Berger J, Fruh A, Spertini F, Bugnon O. Subcutaneous immunoglobulin and support program: what level of interest of patients? Rev Med Suisse. 2015;11(469):831–5.
Ducruet T, Levasseur MC, Des Roches A, Kafal A, Dicaire R, Haddad E. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol. 2013;131(2):585-7 e1-3. doi:10.1016/j.jaci.2012.08.022.
Drummond MF. Sculpher MJ. Torrance GW: O’Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford University Press; 2005.
Henderson K. Training and support to enable home immunoglobulin therapy. Nurs Times. 2003;99(45):28–31.
Gardulf A, Bjorvell H, Andersen V, Bjorkander J, Ericson D, Froland SS, et al. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients’ experiences of subcutaneous self-infusions and home therapy. J Adv Nurs. 1995;21(5):917–27.
Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. BioDrugs. 2007;21(2):105–16.
Bourdin A, Berger J, Perraudin C, Bugnon O. Therapy management program for immunoglobulin self-infusion: patients’ reported outcomes. 43rd European Symposium on Clinical Pharmacy: Patient Safety - Bridging the Gaps; 22–24 October 2014; Copenaghen, Denmark: International Journal of Clinical Pharmacy; 2014. p. p 179-287.
Torgerson TR, Bonagura VR, Shapiro RS. Clinical ambiguities—ongoing questions. J Clin Immunol. 2013;33 Suppl 2:S99–103. doi:10.1007/s10875-012-9851-4.
Author Contributions
All co-authors have reviewed the manuscript and have contributed in a substantive and intellectual manner to the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare having no conflict of interest related to this work. Travel support and unrestricted grant was paid by CSL Behring for facilitating the academic project.
Additional information
Jérôme Berger and Olivier Bugnon are co-senior authors
Rights and permissions
About this article
Cite this article
Perraudin, C., Bourdin, A., Spertini, F. et al. Switching Patients to Home-Based Subcutaneous Immunoglobulin: an Economic Evaluation of an Interprofessional Drug Therapy Management Program. J Clin Immunol 36, 502–510 (2016). https://doi.org/10.1007/s10875-016-0288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-016-0288-z