Abstract
ᅟ
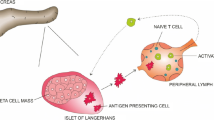
Oral administration of anti-CD3 antibodies induced regulatory T cells (Tregs) alleviating the insulin resistance and liver damage in animal models.
Objective
To determine the safety and biological effects of oral OKT3 monoclonal antibody (Balashov et al. Neurology 55:192–8, 2000) in patients with NASH.
Design
In this Phase-IIa trial, four groups of patients with biopsy-proven NASH (n = 9/group) received placebo (group A) or oral OKT3 (group B: 0.2; C: 1.0; D: 5.0 mg/day) for 30 days. Patients were followed for safety, liver enzymes, glucose, lipid profile, oral glucose tolerance test (OGTT), serum cytokines and Tregs.
Results
Oral OKT3 was well tolerated without treatment-related adverse events. OKT3 induced Tregs: with significant increases of CD4+LAP+ (Latency associated peptide) and CD4+CD25+LAP+ cells in Group D, and a significant increase in TGF-β in Groups C and D. AST decreased significantly in group D and a trend in Groups B and C. Fasting plasma glucose decreased significantly in all treatment groups compared with placebo. OGTT decreased significantly in Group D. Correlations were observed between the changes in several immune-modulatory effects and clinical biomarkers. While serum anti-CD3 levels where undetectable increases in human anti-mouse antibody levels were observed in Groups C and D.
Conclusion
Oral administration of anti-CD3 MAb to patients with NASH was safe and well tolerated. Positive biological effects were noted in several hepatic, metabolic and immunologic parameters. These findings provide the basis for future trials to investigate the effect of oral anti-CD3 MAb immunotherapy in patients with NASH.





Similar content being viewed by others
Abbreviations
- NASH:
-
Nonalcoholic steatohepatitis
- T2D:
-
Type 2 diabetes
- Tregs:
-
Regulatory T cells
- OGTT:
-
Oral glucose tolerance test
- HAMA:
-
Human anti-mouse antibody
- LAP:
-
Latency-associated peptide
- MAb:
-
Monoclonal antibody
- FACS:
-
Fluorescence-activated cell sorting
References
Friend PJ, Hale G, Chatenoud L, et al. Phase I study of an engineered aglycosylated humanized CD3 antibody in renal transplant rejection. Transplantation. 1999;68:1632–7.
Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3 gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–9.
Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8.
Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–32.
Chen Y, Kuchroo VK, Inobe J, et al. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40.
Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14.
Zhang X, Izikson L, Liu L, et al. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53.
Ishikawa H, Ochi H, Chen ML, et al. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–9.
Ochi H, Abraham M, Ishikawa H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med. 2006;12:627–35.
Rav-Acha M, Sassa D, Ilan Y, et al. [Surgical intervention in infective endocarditis: indications and timing]. Harefuah. 2005;144:421–5.
Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–50.
Wu HY, Maron R, Tukpah AM, et al. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J Immunol. 2010;185:3401–7.
Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond). 2008;32 Suppl 7:S52–4.
Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–9.
Ilan Y, Maron R, Tukpah AM, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–70.
Ilan Y, Zigmond E, Lalazar G, et al. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J Clin Immunol. 2010;30:167–77.
Renders L, Valerius T. Engineered CD3 antibodies for immunosuppression. Clin Exp Immunol. 2003;133:307–9.
Weiner HL, da Cunha AP, Quintana F, et al. Oral tolerance. Immunol Rev. 2011;241:241–59.
Meijer RT, Surachno S, Yong SL, et al. Treatment of acute kidney allograft rejection with a non-mitogenic CD3 antibody. Clin Exp Immunol. 2003;133:485–92.
Saharinen J, Hyytiainen M, Taipale J, et al. Latent transforming growth factor-beta binding proteins (LTBPs)–structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev. 1999;10:99–117.
Verma SC, Lan K, Robertson E. Structure and function of latency-associated nuclear antigen. Curr Top Microbiol Immunol. 2007;312:101–36.
Oida T, Zhang X, Goto M, et al. CD4 + CD25- T cells that express latency-associated peptide on the surface suppress CD4 + CD45RB high-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–22.
Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8.
Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16.
Belkaid Y. Regulatory T, cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88.
Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–37.
Shalev I, Schmelzle M, Robson SC, et al. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011;23:282–92.
Bluestone JA, Tang Q. How do CD4 + CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42.
Chatenoud L, Bach JF. Regulatory T cells in the control of autoimmune diabetes: the case of the NOD mouse. Int Rev Immunol. 2005;24:247–67.
Randolph DA, Fathman CG. Cd4 + Cd25+ regulatory T cells and their therapeutic potential. Annu Rev Med. 2006;57:381–402.
von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–94.
Yeh SH, Chuang H, Lin LW, et al. Tai chi chuan exercise decreases A1C levels along with increase of regulatory T-cells and decrease of cytotoxic T-cell population in type 2 diabetic patients. Diabetes Care. 2007;30:716–8.
Ma X, Hua J, Mohamood AR, et al. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology. 2007;46:1519–29.
Chatzigeorgiou A, Chung KJ, Garcia-Martin R, et al. Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology. 2014;60:1196–210.
Acknowledgments
We thank AML (Herzliya, Israel) for clinical chemistry/biochemistry/cytometry analyses and Medistat (Tel Aviv, Israel) for statistical analyses.
Grant Support
This work was supported by NasVax Ltd, Ness-Ziona Israel, and by the Roaman-Epstein Liver Research Foundation.
Disclosure
This clinical trial was funded by NasVax Ltd, Ness-Ziona, Israel; Yaron Ilan is a consultant for NasVax; Nadya Lisovoder, Sarit Samira, and Ronald Ellis are employees and Itamar Shalit is a director of NasVax.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gadi Lalazarv, Meir Mizrahi and Ilit Turgeman contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lalazar, G., Mizrahi, M., Turgeman, I. et al. Oral Administration of OKT3 MAb to Patients with NASH, Promotes Regulatory T-cell Induction, and Alleviates Insulin Resistance: Results of a Phase IIa Blinded Placebo-Controlled Trial. J Clin Immunol 35, 399–407 (2015). https://doi.org/10.1007/s10875-015-0160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0160-6