Abstract
Cytokines play a critical role in the development of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Here we investigated whether IL-27 was elevated in patients with ALI/ARDS and its potential clinical significance. Bronchoalveolar lavage (BAL) and serum samples were obtained from 58 ALI/ARDS patients, and 25 control healthy volunteers. IL-27 and other inflammatory mediators were measured in BAL and serum by ELISA. Besides, a mouse model of cecal ligation and puncture (CLP)-induced lung inflammation/injury was established, and serum, BAL fluid and tissues were collected for analyses in the presence or absence of IL-27 neutralizing antibodies. BAL IL-27 was found to be significantly higher in patients with ALI/ARDS than that in controls, particularly of pulmonary origin; serum IL-27 was also significantly higher. Increased IL-27 was associated with markers of inflammation, and correlated with disease severity of patients in ALI/ARDS. In a mouse model of CLP-induced lung inflammation/injury, elevated IL-27 levels were observed in the lung, serum, and BAL fluids. IL-27 neutralizing antibody treatment reduced pulmonary inflammation and lung injury and improved mouse survival in response to CLP. Therefore, IL-27 is a critical cytokine in ALI/ARDS and inhibition of IL-27 may open a promising approach for ALI/ARDS patients.
Similar content being viewed by others
Introduction
Acute lung injury (ALI),and its more severe form, acute respiratory distress syndrome (ARDS), can develop in many serious conditions including sepsis, pneumonia, major surgery, ischaemia/reperfusion and pancreatitis [1, 2]. The pathogenesis of ALI/ARDS is related to an uncontrolled systemic inflammatory response, microvascular damage, increased pulmonary vascular permeability, interstitial and alveolar oedema, and hypoxaemic respiratory failure. In ALI/ARDS, many inflammatory cytokines are released by leukocytes as well as pulmonary structural cells [3, 4]. A growing body of evidence indicates that excessive production of inflammatory cytokines is critical for the initiation and extension of ALI/ARDS, and the magnitude and duration of this inflammatory process may ultimately determine the outcome in patients with ALI/ARDS [5, 6]. Therefore, understanding of the role of cytokines in ALI/ARDS may result in therapeutic approaches.
Interleukin (IL)-27 is a heterodimeric cytokine composed of the subunit protein IL-27p28 and Epstein Bar virus-induced protein 3 (EBI3) [7]. IL-27 is predominantly expressed by myeloid cells including antigen-presenting cells (APC) upon activation by bacterial and viral pathogens or other non-infectious agents, and it signals through a heterodimeric receptor that consists of T cell cytokine receptor (TCCR/WSX1) and gp130 [8]. Like many cytokines, IL-27 has pleiotropic properties that can limit or enhance ongoing inflammatory responses. Several studies have demonstrated that IL-27 plays an immunoregulatory role in suppressing the development of Th1, Th2, Th17 cell subsets and in inducing anti-inflammatory cytokine IL-10 production [9–11]. Nevertheless, IL-27 exerts a proinflammatory effect in some situations. For example, IL-27 stimulation of monocytes [12], mast cells [8], eosinophils [13] or epithelial cells [14] led to the enhanced production of inflammatory mediators, which would implicates in the exacerbation of inflammatory diseases. Besides, mice deficient for the EBI3 subunit of IL-27 were resistant to experimental septic peritonitis [15], and IL-27 receptor-deficient mice were protected from proteoglycan-induced arthritis and had lower inflammatory reactions and enhanced survival in the MRL/lpr model of lupus [16, 17]. Moreover, IL-27 could promote T cell-dependent colitis [18]. Therefore, IL-27 has important pathophysiological relevance in some inflammatory diseases.
Recent studies showed that TLR2-mediated production of IL-27 by pulmonary epithelial cells promoted bleomycin-induced pulmonary fibrosis in mice [19], and IL-27 directly suppressed endotoxin-induced production of reactive oxygen intermediates by neutrophils and macrophages [15], suggesting a potential role for IL-27 in the development of ALI/ARDS. In this study, we hypothesized that IL-27 levels in patients with ALI/ARDS would be elevated compared to healthy controls. We also sought to determine if IL-27 levels in ALI/ARDS patients would correlate with disease severity and would be associated with increased pulmonary inflammation. Finally, we assessed whether treatment with anti-IL-27 neutralization antibodies could reduce lung inflammatory reactions in a mouse model of cecal ligation and puncture (CLP)-induced lung inflammation/injury.
Materials and Methods
Study Population
Patients were randomly selected from The First Affiliated Hospital of Chongqing Medical University intensive care unit (ICU) patients who had pulmonary edema fluid and plasma samples obtained between 2010 and 2012. Diagnosis of ARDS or ALI was made according to criteria of the American-European Consensus Conference on ARDS [20]. ALI/ARDS was observed after major surgery, multiple trauma, diabetic ketoacidosis, pregnancy induced hypertension, organic phosphorus poisoning, transfusion, pancreatitis, pneumonia, or pulmonary contusion. Exclusion criteria for enrollment of patients were hemofiltration, massive transfusion in the immediately preceding 24 h, medical history of chronic lung diseases, and immunosuppressive therapy. All patients were admitted to ICU at time of ALI/ARDS diagnosis, and they were receiving mechanical ventilation and standard intensive care support. Clinical information on all patients was collected, and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and PaO2/FiO2 ratios were calculated at enrollment (day 1 ALI). The primary outcome, ventilator free days (VFDs), was defined as number of days a patient was alive and free of mechanical ventilation out of 28. ICU free days and mortality (by 21 days) were also recorded. Healthy volunteers without lung diseases who underwent broncho-alveolar lavage (BAL) via bronchoscopy and donated serum served as control subjects. The above protocol was approved by the Clinical Research Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, and informed consent was obtained from all participants according to the Declaration of Helsinki.
BAL and Blood Sampling
BAL fluid and serum were collected from patients and controls as previously described [21]. All patients underwent BAL and had blood collected on the 3rd day after meeting ALI criteria. BAL was centrifuged at 250 g for 10 min at 4 °C, cells were separated, and supernatant was aliquoted into small volumes. The standardization of cytokine levels in the BAL fluids was based on the concept that aliquots of sterile normal saline infused through the bronchoscope mix with epithelial lining fluid (ELF). When the saline was recovered by aspiration, the ELF and its components were recovered along with it. BAL cytokine concentration was adjusted using urea. For quantification of alveolar lining fluid, urea was used as an endogenous marker of dilution. Values were corrected according to the dilution factor as ratio of serum urea/BAL urea. To obtain differential cell counts, a 100 μL aliquot of cells was subjected to cytocentrifugation (Cytospin; Cytopro Wescor; Syracuse, NY), air dried, and stained with Giemsa Diff-Quik II stain (Baxter Scientific Products; McGaw Park, IL). Differential cell counts were made from a minimum count of 300 cells and results were expressed as a proportion of total cell population. Cell counts were thus expressed for neutrophils, macrophages, and lymphocytes. Blood was similarly centrifuged to separate cells from serum immediately after collection and aliquoted. All serum and BAL supernatant samples were stored at −80 °C.
Human BAL and Serum Analysis
IL-27 in BAL and serum was measured by ELISA (R & D Systems, Inc, Minneapolis, MN) in duplicate. A panel of cytokines including IL-1β, IL-2, IL-6, IL-18, IFN-γ, TNF-α, CXCL8 and CXCL10 were also measured in BAL and serum samples using ELISA kits (Biolegend) in duplicate.
Mouse Experiments
Eight- to fourteen-week-old C57BL/6 mice were obtained from and raised at Chongqing Medical University. All animal experiments were done in accordance with the Institutional Animal Care and Use Committee’s guidelines at the Chongqing Medical University. The surgical procedure to establish CLP-induced sepsis was performed as previously described [22]. In brief, mice were lightly anesthetized with ketamine and xylazine hydrochloride by intraperitoneal injection, and a middle abdominal incision was made. The cecum was delivered, ligated with a silk suture 1 cm from the tip, and doubly punctured with a 21-gauge needle. After puncture, the cecum was gently squeezed to extrude a small amount of feces and returned to the abdominal cavity. The laparotomy was then closed. Additionally, sham-operated control mice were subjected to the same surgical laparotomy after anesthesia, but the cecum was neither ligated nor punctured. Mice were monitored for 7 days after CLP. For survival analysis, mice were monitored twice daily for 21 days.
Mouse IL-27 Neutralizing Antibody Treatment
We pretreated C57Bl/6 mice with 50 μg of either goat isotype Immunoglobulin-G (IgG) or polyclonal goat anti-mouse IL-27 antibodies (R&D Systems) 1 h prior to experimental manipulations. Mice inhaled the compounds diluted in 10 μl of normal saline, and serum, BAL fluid, lung and survival analysis were then performed at predetermined time points.
Quantitative Analysis of Gene Expression
Total cellular RNA was extracted with RNeasy columns (QIAGEN), and quantitative real-time PCR analysis for mouse IL-27 EBI3 and p28, was performed using specific primers. EBI3, sense, 5′-ACC CAT TGA AGC CAC GAC TT-3′, and antisense, 5′-AGT ATT GCA TCC AGG TGT CAG CT-3′; p28, sense, 5′-CTC TGC TTC CTC GCT ACC AC-3′, and antisense, 5′-GGG GCA GCT TCT TTT CTT CT-3′. Real-time PCR was performed on an ABI PRISM 7000 (Applied Biosystems) using SYBR Green quantitative PCR (Roche Diagnostics) according to the manufacturers’ instructions.
Mouse Serum and BAL Fluid Analysis
At the indicated times, mice were sacrificed and blood was drawn by puncturing the left ventricle, and the serum was separated by centrifuging the blood samples. Mouse BAL fluid was isolated and spun to separate cellular components. The cell pellet was used for quantitative and qualitative cell counts, and lavage supernatant was collected. Finally, mouse serum and BAL fluid were used for quantitative cytokine analysis including IFN-γ, IL-2, IL-6, KC, IL-10, IL-18, CXCL8 and CXCL10 using ELISA kits (Biolegend).
Histologic Examination
In a separate set of experiments, mouse left lungs were obtained from sham-operated or septic animals and submerged in 10 % Formalin in neutral buffered solution (Sigma, St. Louis, MO) for immediate fixation and later embedded in paraffin. Lung tissues were stained with hematoxylin and eosin (H&E), dehydrated, and coverslipped. Morphologic examinations were performed by light microscopy and documented by photographs.
Statistical Analysis
All data were expressed as either mean ± standard deviation or median values and interquartile ranges. Differences between groups were assessed by Mann–Whitney U test, Student’s t-test or Kruskal–Wallis test followed by Dunn’s test. Non-parametric Spearman’s Rank Correlation Coefficient was used to test correlations between two parameters. Kaplan–Meier survival analysis was used for the survival experiments. A probability (p) < 0.05 was considered significantly different.
Results
Patient Characteristics
A total of 83 subjects (58 patients with ALI/ARDS, and 25 healthy controls) were recruited in this study, and the clinical characteristics of subjects were summarized in Table I. All patients met ALI PaO2/FiO2 criteria with a mean PaO2/FiO2 ratio of 131.6 (±55.2). The average age was 46 years and 22/58 of the patients were female. The cause of ARDS/ALI was direct lung injury in 27 patients (related to pneumonia, n = 25; pulmonary contusion, n = 2) and indirect lung injury in 31 patients (related to major surgery, n = 3; multiple trauma, n = 5; diabetic ketoacidosis, n = 3; pregnancy induced hypertension, n = 6; organic phosphorus poisoning, n = 3; transfusion, n = 1; pancreatitis, n = 10). The average enrollment APACHE II score was 17.8 (±8.5). The average Ventilator Free Days (VFDs) were 12.5 (±8.5) and ICU free days were 8.5 (±7.5).
IL-27 in BAL Fluid and Serum was Elevated in ALI/ARDS
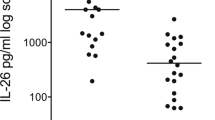
BAL levels of IL-27 in patients with ALI/ARDS were significantly higher than in controls (p < 0.001, Fig. 1a). Similarly, serum levels of IL-27 in patients were also significantly higher than in controls (p < 0.001, Fig. 1b). However, there was no significant correlation between serum and BAL fluid concentrations for individual patients (r = 0.366, p = 0.052). Besides, IL-27 concentrations were significantly higher in BAL fluids from patients with pulmonary ALI/ARDS than those from patients with lung injury of non-pulmonary cause (p < 0.01, Fig. 1c), and there was also a significant difference in BAL fluid levels of IL-27 between the control and non-pulmonary groups (p < 0.05). In contrast, serum levels of IL-27 did not differ between patients with ALI/ARDS of direct and indirect aetiology (p > 0.05, Fig. 1d). 6 patients with ALI/ARDS were recruited after treatment by week 8, and BAL concentrations of IL-27 decreased and the percent of change in IL-27 values was more than 25 % (Fig. 1e), and IL-27 levels in serum showed similar kinetics with those observed in BAL (Fig. 1f).
IL-27 concentrations were elevated in patients with ALI/ARDS. a BAL and b serum IL-27 levels were measured by ELISA. c BAL and d serum levels of IL-27 from patients with ALI/ARDS of pulmonary aetiology and extrapulmonary aetiology. Six patients with ALI/ARDS were also recruited after 8 weeks of treatments to investigate e BAL and f serum concentrations of IL-27. The Mann–Whitney rank sum test was used to assess the differences of concentration of IL-27
From our ROC analysis, a cut-off level of BAL IL-27 for the diagnosis of ALI/ARDS has been set to 9.1 ng/ml. The specificity, sensitivity, positive predictive value and negative predictive value were 91 %, 36 %, 92 % and 57 %, respectively.
Relationship Between BAL IL-27, and BAL Cell Counts or Cytokines in ALI/ARDS
As shown in Fig. 2a, a significant correlation was detected between BAL IL-27 levels and BAL macrophages (r = 0.414, P < 0.05), neutrophils (r = 0.446, P < 0.05) or lymphocytes (r = 0.594, P < 0.01) in patients with pulmonary ALI/ARDS. And a similar association between BAL IL-27 and cellular infiltration was also found in patients with lung injury of non-pulmonary cause (Fig. 2b). In addition, BAL IL-27 was positively and significantly correlated with TNF-α (r = 0.383, P < 0.05, Fig. 2c) and CXCL10 (r = 0.282, P < 0.05, Fig. 2d). While increases in BAL IL-27 was associated with increases in BAL IL-1β, IL-6, IL-18 or CXCL8, but this did not reach statistical significance (P > 0.08 for IL-1β, P > 0.05 for IL-6, P > 0.06 for IL-18 and P > 0.1 for CXCL8).
a Correlation between BAL IL-27 levels and BAL macrophages, neutrophils or lymphocytes in patients with pulmonary ALI/ARDS. b Correlation between BAL IL-27 levels and BAL macrophages, neutrophils or lymphocytes in patients with lung injury of non-pulmonary cause. c Correlation between BAL IL-27 and TNF-α in all patients with ALI/ARDS. d Correlation between BAL IL-27 and CXCL10 in all patients with ALI/ARDS. The nonparametric Spearman rank correlation test was used to test correlations between two parameters
Relationship Between IL-27 and ALI Severity
As shown in Fig. 3, there was a significant correlation between severity of illness assessed by APACHE II score and IL-27 levels in either BAL fluid (r = 0.402, P < 0.05, Fig. 3a) or serum (r = 0.297, P < 0.05, Fig. 3b) of ALI/ARDS patients. In addition, higher levels of BAL or serum IL-27 were associated with worse clinical outcomes, including higher hospital mortality (Fig. 3c and d) and a shorter duration of unassisted ventilation (Fig. 3e and f).
Correlation between a BAL or b serum IL-27 levels and disease severity in patients with ALI/ARDS. APACHE II scores were calculated from data collected during the 24 h period in which the bronchoscopy was performed. The nonparametric Spearman rank correlation test was used to test correlations between two parameters. c BAL and d serum IL-27 levels in survived and dead patients with ALI/ARDS. e BAL and f serum IL-27 levels in patients with ALI/ARDS who received unassisted ventilation over a 28-day period
IL-27 Production was Increased in a Mouse Model of CLP-Induced Lung Inflammation/Injury
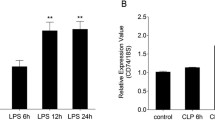
To further study IL-27 expression in the lung, a mouse model of CLP-induced lung inflammation/injury was established. We found that EBI3 and p28 mRNA levels in the lung were substantially increased 6 h after CLP, and the EBI3 and p28 mRNA levels still remained up-regulated 24 h after CLP (Fig. 4a). Besides, we detected a strong up-regulation of IL-27 protein in BAL fluid after CLP (Fig. 4b). Similarly, increase in IL-27 mRNA level was detected in the peripheral blood mononuclear cells (PBMC) (Fig. 4c), and IL-27 protein production was also elevated in serum (Fig. 4d).
IL-27 levels were elevated in CLP-induced ALI/ARDS. C57BL/6 mice (n = 6) were subjected to sham or CLP surgery. a Lungs were removed for total RNA extraction at the indicated time points, and quantitative real-time PCR was performed. Relative expression levels of the genes were expressed with β-actin housekeeping gene as internal reference. b The concentrations of IL-27 in BAL fluid 24 h after CLP. c The mRNA expression of IL-27 subunits in PBMC at the indicated time points after CLP. d The concentrations of IL-27 in serum 24 h after CLP. ***P < 0.001 when compared between groups denoted by horizontal lines
Effect of IL-27 Neutralizing Antibody Treatment on CLP-Induced Lung Inflammation/Injury
We further tested whether exogenous IL-27 inhibition can prevent CLP-induced lung inflammation/injury. Mice inhaled a single dose of IL-27 neutralizing antibodies or an equal dose of control IgG 1 h prior to CLP, and lung injury indices were compared. Histologic assessment of representative lung sections from mice with CLP-induced sepsis revealed evidence of lung injury, including edema, hyperemia and congestion, leukocytes margination and tissue infiltration, intraalveolar hemorrhage and debris, and cellular hyperplasia, whereas inflammation was decreased in mice that received IL-27 neutralizing antibody (Fig. 5a). Besides, IL-27 neutralizing antibody treatment significantly decreased macrophages (P < 0.05), neutrophils (P < 0.05) or lymphocytes (P < 0.05) cell counts in BAL fluid (Fig. 5b), and this treatment also significantly reduced the levels of pulmonary cytokines and chemokines measured, including IFN-γ, IL-2, IL-6, KC, IL-10, IL-18, CXCL8 and CXCL10 (P < 0.05 in all cases, Fig. 5c). Moreover, the survival rate of mice treated with anti-IL-27 neutralizing antibodies was significantly increased compared with isotype control-treated mice (Fig. 5d).
IL-27 neutralizing antibodies attenuated CLP-induced ALI/ARDS. C57BL/6 mice (n = 8) inhaled 50 μg of either goat isotype IgG or polyclonal goat anti-mouse IL-27 antibodies, and mice were then subjected to CLP-induced lung inflammation/injury. a Hematoxylin and eosin stained lung histology from representative mice 24 h after the CLP procedure (40×). b Cell counts including macrophages, neutrophils and lymphocytes were performed on BAL fluid to evaluate lung inflammation 24 h after CLP. c Levels of cytokines and chemokines including IFN-γ, IL-2, IL-6, KC, IL-10, IL-18, CXCL8 and CXCL10 in BAL fluid 24 h after CLP. d The mice survival rates were monitored for 21 days after CLP. *P < 0.05 when compared between groups denoted by horizontal lines
Discussion
This study demonstrated that IL-27 was detectable in BAL and serum from patients with ALI/ARDS and was elevated compared to healthy controls. Besides, IL-27 correlated with the numbers of macrophages, neutrophils and lymphocytes in BAL fluids, and it was associated with the levels of some inflammatory cytokines and chemokines. Moreover, the level of IL-27 was a potential predictor of disease severity of ALI/ARDS. Our results thus provide the first evidence that IL-27 plays an important role in promoting the massive pulmonary inflammation during ALI/ARDS.
The immunopathological role of IL-27 has been described in some inflammatory diseases. It has been suggested that IL-27 functioned by inducing the differentiation of IFN-γ-producing T cells in vivo, which was essential for the development of arthritis in a proteoglycan-induced arthritis model [16]. In the ConA model of T cell-mediated hepatitis, IL-27EBI3-deficient mice were found to be almost completely protected from ConA-induced liver damage [23]. Besides, IL-27 also contributed to T cell–dependent intestinal inflammation and it was suggested that IL-27 would be a novel potential therapeutic target for treatment of sepsis [15, 18]. The proinflammatory role of IL-27 in airway inflammatory diseases has been recently identified. Our previous studies have claimed that the levels of IL-27 were elevated in airway inflammatory diseases, such as chronic obstructive pulmonary diseases (COPD) and pulmonary tuberculosis [24], and a more recent study also demonstrated elevated IL-27 levels in patients with influenza A virus infection [25]. Furthermore, IL-27 could activate pulmonary epithelial cells to produce IL-6, CXCL10 and intercellular adhesion molecule 1 (ICAM-1), and it could induce human monocytes, mast cells and eosinohphils to produce a variety of cytokines and chemokines, including IL-6, IL-18, TNF-α, MIP-1α, and MIP-1β, IL-1β, CXCL1, CCL2 and CXCL8, singly or jointly [8, 12–14, 24]. All these target cells of IL-27 are potential effector cells in local airway inflammatory reactions, IL-27 may thus play a critical role in airway inflammation, which triggers or progresses to AIL.
IL-27 is known to be mainly expressed by APC, such as dendritic cells [26], macrophages [27] and monocytes [28], which could be accumulated and activated during ALI. Thus, it is logical that IL-27 might serve as an indicator in ALI. In fact, our present study showed that there was an association between IL-27 concentrations and macrophages in BAL fluids. Besides, BAL IL-27 also correlated with BAL neutrophils and lymphocytes, and this could be explained by that IL-27 is an inducer of CXCL8 and CXCL10, which are potent chemokines for neutrophils and activated T cells, respectively [29]. Furthermore, BAL IL-27 had a relationship with some cytokines and chemokines. All these data support the proinflammatory role of IL-27 in ALI. For the diagnosis of ALI by IL-27, the specificity, sensitivity, positive predictive value and negative predictive value were 91 %, 36 %, 92 % and 57 %, respectively. The limitation of IL-27 as a biomarker should be considered. BAL IL-27 levels of only 18 out of 58 ALI/ARDS patients were higher than the maximum BAL IL-27 levels of healthy controls. This means that BAL IL-27 may not always be useful to differentiate healthy controls from ALI/ARDS patients. Besides, although regression analysis of APACHEII scores and IL-27 levels was shown to be significantly correlated, the slope of the regression line was too small to be of any clinical significance. Thus it is underpowered to detect an association between disease severity and IL-27 in this small population of lung injury patients. Samples collected over multiple time points from a large and diverse population of lung injury patients are required to further determine the utility of IL-27 as a diagnostic and prognostic biomarker for ALI/ARDS. However, effective treatments could down-regulate increased levels of IL-27 in patients with ALI/ARDS, supporting IL-27 as pathophysiologic process in ALI.
The lung is one of the first organs to be affected in septic patients, to further study the role of the IL-27 in ALI, we used a mouse model of CLP-induced lung inflammation/injury. Our present study showed that lung injuries, characterized by increased disruption of lung architecture, extravasation of red blood cells, accumulation of inflammatory cells, and interstitial edema, were present 24 h after CLP in vehicle-treated animals. These injuries were associated with increased levels of IL-27 in the pulmonary tissues and circulating blood. Interestingly, the expression of IL-27 has been strongly increased as early as 6 h in the lungs or PBMC after CLP, suggesting that IL-27 production may be an initial event in the inflammatory process of AIL/ARDS. In this regard, inhibition of IL-27 may be a useful adjunct in the treatment of CLP-induced lung inflammation/injury. Here, we demonstrated that treatment with IL-27 neutralizing antibodies decreased the levels of pulmonary inflammatory cytokine/chemokine which has been shown to play an important role in the development of ALI/ARDS [26, 29, 30], and prevented lung edema and inflammatory cell recruitment to the airways after CLP-induced lung inflammation/injury. Furthermore, inhibition of IL-27 could significantly improve mice survival after CLP-induced lung inflammation/injury. It is now well considered that an integrated network of inflammatory mediators and a variety of inflammatory cells plays pivotal role in the pathogenesis of AIL/ARDS [1, 3], our results therefore suggest that these beneficial effects of IL-27 inhibition principally involve the prevention of excessive inflammatory reactions during ALI/ARDS. A previous study also showed that in vivo blockade of IL-27 function using a soluble IL-27 receptor fusion protein led to reduced pSTAT1 levels and suppression of liver injury [23]. All these work support a key pathogenic role of IL-27 in inflammation-mediated tissue injury. Anyway, this is the first report that IL-27 may be a novel therapeutic target in treating ALI/ARDS.
It should be notified that although neutralization of IL-27 has a beneficial effect in a mouse model of CLP-induced lung inflammation/injury, it may reflect a role of IL-27 in mediating early inflammatory changes triggered by septic peritonitis that ultimately result in lung injury as opposed to playing a direct role in triggering functional disruption of lung function. Thus, further studies are required to investigate the protective effects of neutralizing IL-27 in other models that directly target lung, such as ventilator-induced or virus-induced lung injury. Besides, a recent study also showed that IL-18 neutralizing antibody treatment could reduce lung injury in response to mechanical ventilation [30]. We speculate that the combination of neutralizing antibodies against IL-27 and IL-18 or other inflammatory mediators would offer the potential of targeting multiple inflammatory mechanisms, thereby improving the effectiveness for the treatment of ALI, and this way warrants further investigation.
In conclusion, IL-27 levels were raised in BAL fluids and serum of patients with ALI/ARDS, and increases in IL-27 correlated with inflammatory reaction and disease severity in patients with ALI/ARDS. Moreover, we demonstrated that neutralization of IL-27 decreased CLP-induced lung inflammation/injury in a mouse model, suggesting that IL-27 might be a potential therapeutic target in ALI/ARDS, a disease which continues to have a high mortality.
Change history
10 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10875-023-01533-4
References
Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63.
Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93.
Perl M, Lomas-Neira J, Venet F, et al. Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011;5:115–26.
Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–6.
Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99.
Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–57.
Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–47.
Pflanz S, Hibbert L, Mattson J, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–31.
Yoshida H, Nakaya M, Miyazaki Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol. 2009;86:1295–303.
Hirahara K, Ghoreschi K, Yang XP, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–30.
Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–7.
Guzzo C, Ayer A, Basta S, et al. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J Immunol. 2012;188:864–73.
Hu S, Wong CK, Lam CW. Activation of eosinophils by IL-12 family cytokine IL-27: implications of the pleiotropic roles of IL-27 in allergic responses. Immunobiology. 2011;216:54–65.
Cao J, Wong CK, Yin Y, et al. Activation of human bronchial epithelial cells by inflammatory cytokines IL-27 and TNF-alpha: implications for immunopathophysiology of airway inflammation. J Cell Physiol. 2010;223:788–97.
Wirtz S, Tubbe I, Galle PR, et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–81.
Cao Y, Doodes PD, Glant TT, et al. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–30.
Sugiyama N, Nakashima H, Yoshimura T, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of interleukin 27 receptor alpha (WSX-1). Ann Rheum Dis. 2008;67:1461–7.
Cox JH, Kljavin NM, Ramamoorthi N, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–23.
Kim HS, Go H, Akira S, et al. TLR2-mediated production of IL-27 and chemokines by respiratory epithelial cells promotes bleomycin-induced pulmonary fibrosis in mice. J Immunol. 2011;187:4007–17.
Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24.
Callister ME, Burke-Gaffney A, Quinlan GJ, et al. Extracellular thioredoxin levels are increased in patients with acute lung injury. Thorax. 2006;61:521–7.
Lomas-Neira JL, Chung CS, Grutkoski PS, et al. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64.
Siebler J, Wirtz S, Frenzel C, et al. Cutting edge: a key pathogenic role of IL-27 in T cell-mediated hepatitis. J Immunol. 2008;180:30–3.
Cao J, Zhang L, Li D, Xu F, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest. 2012;141:121–30.
Liu L, Cao Z, Chen J, et al. Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. J Biol Chem. 2012;287:11899–910.
Kim JH, Chung DH. CD1d-restricted IFN-γ-secreting NKT cells promote immune complex-induced acute lung injury by regulating macrophage-inflammatory protein-1α production and activation of macrophages and dendritic cells. J Immunol. 2011;186:1432–41.
Johnston LK, Rims CR, Gill SE, et al. Pulmonary macrophage subpopulations in induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–26.
Wilson MR, O’Dea KP, Zhang D, et al. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2009;179:914–22.
Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp Cell Res. 2011;317:575–89.
Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–34.
Acknowledgments
This study is supported by National Natural Science Foundation grants of China (No, 81200054 and 81000711).
Declaration of Interest
No authors have a conflict of interest with organizations with financial interest in the subject matter.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, F., Liu, Q., Lin, S. et al. IL-27 is Elevated in Acute Lung Injury and Mediates Inflammation. J Clin Immunol 33, 1257–1268 (2013). https://doi.org/10.1007/s10875-013-9923-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9923-0