Abstract
Purpose
T-helper (Th) cells abnormalities are considered to be associated with the pathogenesis of Systemic lupus erythematosus (SLE). Recently, The Th22 cells have been identified and implicated in the pathogenesis of autoimmune diseases such as Rheumatoid arthritis (RA), although therir role in Systemic lupus erythematosus (SLE) remains unclear. The present study intends to investigate their roles in SLE.
Methods
Clinical data were collected in 65 SLE patients and 30 healthy controls. The patients were divided into active and inactive groups. CD4+IFN-γ−IL-17−IL-22+Tcells (Th22 cells),CD4+ IFN-γ−IL-22−IL-17+T cells (Th17 cells),and CD4+ IFN-γ+ (Th1 cells) were assayed by flow cytometry. Serum interleukin-22 (IL-22) and IL-17 levels were measured by enzyme-linked immunosorbent assay.
Results
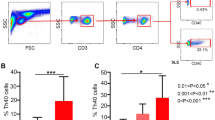
The main observation focused on increased Th22 cells in patients with sole lupus skin disease and decreased Th22 cells in patients with sole lupus nephritis. Likewise, concentrations of serum IL-22 were increased in patients with sole lupus skin disease, and decreased in patients with sole lupus nephritis. Additionally, there was a positive correlation between the percentage of Th22 cells and IL-22 production. The percentage of Th17 cells or concentration of serum IL-17 correlated positively with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
Conclusion
Th22 seems to be a more significant index to predict the tissue involvement of SLE than Th17, although Th17 may play a role in the activity of SLE.





Similar content being viewed by others
References
Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73.
Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9.
Wedderburn LR, Robinson N, Patel A, Varsani H, Woo P. Selective recruitment of polarized T cells expressing CCR5 and CXCR3 to the inflamed joints of children with juvenile idiopathic arthritis. Arthritis Rheum. 2000;43:765–74.
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–4.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41.
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–7.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. New Engl J Med. 2009;361:888–98.
Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–6.
Sullivan KE, Piliero LM, Dharia T, Goldman D, Petri MA. 3- polymorphisms of ETS1 are associated with different clinical phenotypes in SLE. Hum Mutat. 2000;16:49–53.
Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory Cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–93.
Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–75.
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63.
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subsetinvolved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85.
Trifari S, Kaplan C, Tran E, Crellin N, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71.
Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related Tcell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–9.
Zhang N, Pan H-F, Ye D-Q. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem. 2011;353:41–6.
Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83.
Truchetet ME, Brembillal NC, Montanari E, et al. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13:R166.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56.
Zhang L, Li JM, Liu XG, et al. Elevated Th22 Cells Correlated with Th17 Cells in Patients with Rheumatoid Arthritis. J Clin Immunol. 2011;31:606–14.
Ikeuchi H, Kuroiwa T, Hiramatsu N, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52(4):1037–46.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Gladman DD, Ibanez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol. 2002;29:288–91.
Pan HF, Fnag XH, Wu GC, et al. Anti-neutrophil cytoplasmic antibodies in new-onset Systemic Lupus Erythematosus and lupus nephritis. Inflammation. 2008;31:260–5.
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54.
Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31.
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–57.
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51.
Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607.
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87(5):523–36.
Kolb H, Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992;13:157–1601.
Jonuleit H, Dnop J, End AH. Cytokines and their effects on maturation, differentiation and migration of dendratic cells. Arch Dermatol Res. 1996;289:1.
Fairhurst AM, Mathian A, Connolly JE, et al. Systemic TNF-αdrives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008;38(7):1948–60.
Jacob CO, Fronek Z, Lewis GD, et al. Heritable major histocompatibility complex class II-associated difference in production of tumor necrosis factor alpha: relevance to genetic predisposition to systemic lupus erythematosus. Proc Nacl Acad Sci U S A. 1990;87:1233–7.
Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–85.
Dong G, Ye R, Shi W, et al. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl). 2003;116:543–8.
Ma J, Yu J, Tao X, et al. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–8.
Henriques A, Ines L, Couto M, et al. Frequency and functional activity of Th17, Tcl7 and other T-cell subsets in Systemic Lupus Erythematosus. Cell Immunol. 2010;264:97–103.
Funauclli MS, Ikoma H, Enomoto, et al. Decreased Thl-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol. 1998;27(3):p219–24.
Chang DM, Su WL, Chu SJ. The expression and significance of intracellular T helper cytokines in systemic 1upus erythematosus. Immunol Invest. 2002;31(1):1–12.
Acknowledgments
This study was partially supported by grant from Science Foundation of Science and Technology Department of Zhejiang Province (No.2007C3305) and (No.2009C3012-4).
Disclosures
The authors have no financial conflict of interest.
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Xy., Wang, Hy., Zhao, Xy. et al. Th22, but not Th17 Might be a Good Index to Predict the Tissue Involvement of Systemic Lupus Erythematosus. J Clin Immunol 33, 767–774 (2013). https://doi.org/10.1007/s10875-013-9878-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9878-1