Abstract
Objective
The pathogenesis of hepatitis B virus (HBV)-associated chronic liver disease is still not fully understood. The immune imbalance of cytokine profile exerts a profound influence on the resolution of HBV infections and HBV clearance. This present study aimed to evaluate the immune status of the peripheral T helper (Th) 17 and Th1 cells in the active patients with chronic HBV infection.
Materials and Methods
Thirty patients with chronic active hepatitis B were included in our present study. The frequency of peripheral Th 17 cells (CD3+CD8−IL-17+ T cells), Th1 cells (CD3+CD8−IFN-γ+ T cells), and Tc1 cells (CD3+CD8+IFN-γ+ T cells) in chronic hepatitis B (CHB) were analyzed by flow cytometry. The protein and mRNA levels of interleukin-17 (IL-17) and interferon-gamma (IFN-γ) were measured by enzyme-linked immunosorbent assay and quantitative real-time polymerase chain reaction (PCR).
Results
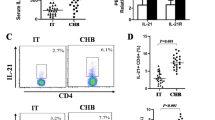
The percentage of Th17 cells in peripheral blood of CHB patients (1.53 ± 0.52%) was significantly increased than that in normal controls (0.92 ± 0.20%; P < 0.05). In contrast, the percentage of Th1 and Tc1 cells of CHB patients was significantly decreased as compared with that of control group. The frequency of Th17 cells had a negative correlation with Th1 cells, and a positive correlation with serum alanine aminotransferase in CHB patients.
Conclusion
The elevated peripheral Th17 cells were obtained in the patient with chronic active hepatitis B, suggesting its potential role in the immune activation of chronic HBV infection.






Similar content being viewed by others
References
Lok AS. The maze of treatments for hepatitis B. N Engl J Med. 2005;352:2743–6.
Kägi D, Ledermann B, Bürki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–32.
Vierling JM. The immunology of hepatitis B. Clin Liver Dis. 2007;11:727–59.
Klein J, Sato A. The HLA system. First of two parts. N Engl J Med. 2000;343:702–9.
Klein J, Sato A. The HLA system. Second of two parts. N Engl J Med. 2000;343:782–6.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41.
Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67.
Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48.
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33.
Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188–211.
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76.
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40.
Ma D, Zhu X, Zhao P, Zhao C, Li X, Zhu Y, et al. Profile of Th17 cytokines (IL-17, TGF-beta, IL-6) and Th1 cytokine (IFN-gamma) in patients with immune thrombocytopenic purpura. Ann Hematol. 2008;87:899–904.
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–7.
Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–57.
Penna A, Del Prete G, Cavalli A, Bertoletti A, D’Elios MM, Sorrentino R, et al. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022–7.
Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–9.
Ferrari C, Missale G, Boni C, Urbani S. Immunopathogenesis of hepatitis B. J Hepatol. 2003;39(Suppl 1):S36–S42.
Löhr HF, Krug S, Herr W, Weyer S, Schlaak J, Wölfel T, et al. Quantitative and functional analysis of core-specific T-helper cell and CTL activities in acute and chronic hepatitis B. Liver. 1998;18:405–13.
Kondo Y, Kobayashi K, Ueno Y, Shiina M, Niitsuma H, Kanno N, et al. Mechanism of T cell hyporesponsiveness to HBcAg is associated with regulatory T cells in chronic hepatitis B. World J Gastroenterol. 2006;12:4310–7.
Akpolat N, Yahsi S, Godekmerdan A, Demirbag K, Yalniz M. Relationship between serum cytokine levels and histopathological changes of liver in patients with hepatitis B. World J Gastroenterol. 2005;11:3260–3.
Jiang R, Feng X, Guo Y, Lu Q, Hou J, Luo K, et al. T helper cells in patients with chronic hepatitis B virus infection. Chin Med J (Engl). 2002;115:422–4.
Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50.
Shahrara S, Huang Q, Mandelin AM 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93.
Prussin C. Cytokine flow cytometry: understanding cytokine biology at the single-cell level. J Clin Immunol. 1997;17:195–204.
Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30.
Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810.
Bertoletti A, Costanzo A, Chisari FV, Levrero M, Artini M, Sette A, et al. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J Exp Med. 1994;180:933–43.
Yan MX, Mao HT, Liu Q, Wang WQ, Li YQ. Elevated levels of serum soluble E-selectin in patients with chronic hepatitis B: correlation with T lymphocyte subsets, NK cells and liver inflammation. Hepatol Res. 2006;35:111–7.
Tang TJ, Kwekkeboom J, Laman JD, Niesters HG, Zondervan PE, de Man RA, et al. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10:159–67.
Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70.
Xu Y, Du WJ, Qin LY, Xing ZZ, Qin XH, Chen SJ. Expression of interleukin-17 in hepatitis B related liver fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:133–5.
Acknowledgments
This work was supported by grants from Key Project of Chinese Ministry of Science and Technology (2008ZX10002-007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, J., Wang, K., Meng, QH. et al. Implication of Th17 and Th1 Cells in Patients with Chronic Active Hepatitis B. J Clin Immunol 30, 60–67 (2010). https://doi.org/10.1007/s10875-009-9328-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9328-2