Abstract
Introduction
Autoinflammatory diseases are a group monogenic inflammatory conditions characterized by an early onset during childhood.
Discussion
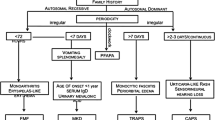
Under the term “periodic fevers” are gathered some monogenic diseases (familial Mediterranean fever, mevalonate kinase deficiency, and tumor necrosis factor receptor-associated syndrome) characterized by periodic or recurrent episodes of systemic inflammation causing fever often associated with rash, serositis (peritonitis, pleuritis), lymphadenopathy, arthritis, and other clinical manifestations. Systemic reactive (AA) amyloidosis may be a severe long-term complication. Cryopyrinopathies are a group of conditions associated to mutations of the gene Cryopyrin that are responsible for a spectrum of diseases (familial cold autoinflammatory syndrome, Muckle–Wells syndrome, and chronic infantile neurological cutaneous and articular syndrome) characterized by a chronic or recurrent systemic inflammation variably associated with a number of clinical features, such as urticarial-like rash, arthritis, sensorineural deafness, and central nervous system and bone involvement. Other disorders are dominated by the presence of sterile pyogen abscesses prevalently affecting the skin, joints, and bones (pyogenic disorders). These include pyogenic sterile arthritis, pyoderma gangrenosum, and acne syndrome, and Majeed syndrome. Finally, some diseases, such as Blau’s syndrome, are characterized by the appearance of typical noncaseating granulomatous inflammation affecting the joints, skin, and uveal tract (granulomatous disorders). In the present review, we will focus on the clinical presentation of these disorders in childhood and report on the available therapeutic strategies.


Similar content being viewed by others
References
The French FMF Consortium. A candidate gene for familial Mediterranean fever. The French FMF Consortium. Nat Genet 1997;17(1):25–31.
The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell 1997;90(4):797–807.
Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine (Baltimore) 2005;84(1):1–11.
Gedalia A, Adar A, Gorodischer R. Familial Mediterranean fever in children. J Rheumatol Suppl 1992;35:1–9.
Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum 1997;40(10):1879–85.
Touitou I, Lesage S, McDermott M, Cuisset L, Hoffman H, Dode C, et al. Infevers: an evolving mutation database for auto-inflammatory syndromes. Human Mutat 2004;24(3):194–8.
Touitou I, Sarkisian T, Medlej-Hashim M, Tunca M, Livneh A, Cattan D, et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum 2007;56(5):1706–12.
Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med 1972;287(25):1302.
Kallinich T, Haffner D, Niehues T, Huss K, Lainka E, Neudorf U, et al. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics 2007;119(2):e474–83.
Belkhir R, Moulonguet-Doleris L, Hachulla E, Prinseau J, Baglin A, Hanslik T. Treatment of familial Mediterranean fever with anakinra. Ann Intern Med 2007;146(11):825–6.
van der Meer JW, Vossen JM, Radl J, van Nieuwkoop JA, Meyer CJ, Lobatto S, et al. Hyperimmunoglobulinaemia D and periodic fever: a new syndrome. Lancet 1984;1(8386):1087–90.
Houten SM, Kuis W, Duran M, De Koning TJ, van Royen-Kerkhof A, Romeijn GJ, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet 1999;22(2):175–7.
Hoffmann GF, Charpentier C, Mayatepek E, Mancini J, Leichsenring M, Gibson KM, et al. Clinical and biochemical phenotype in 11 patients with mevalonic aciduria. Pediatrics 1993;91(5):915–21.
Frenkel J, Houten SM, Waterham HR, Wanders RJ, Rijkers GT, Duran M, et al. Clinical and molecular variability in childhood periodic fever with hyperimmunoglobulinaemia D. Rheumatol (Oxf) 2001;40(5):579–84.
D’Osualdo A, Picco P, Caroli F, Gattorno M, Giacchino R, Fortini P, et al. MVK mutations and associated clinical features in Italian patients affected with autoinflammatory disorders and recurrent fever. Eur J Hum Genet 2005;13(3):314–20.
Simon A, Kremer HP, Wevers RA, Scheffer H, De Jong JG, van der Meer JW, et al. Mevalonate kinase deficiency: evidence for a phenotypic continuum. Neurology 2004;62(6):994–7.
Takada K, Aksentijevich I, Mahadevan V, Dean JA, Kelley RI, Kastner DL. Favorable preliminary experience with etanercept in two patients with the hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis Rheum 2003;48(9):2645–51.
Marchetti F, Barbi E, Tommasini A, Oretti C, Ventura A. Inefficacy of etanercept in a child with hyper-IgD syndrome and periodic fever. Clin Exp Rheumatol 2004;22(6):791–2.
Cailliez M, Garaix F, Rousset-Rouviere C, Bruno D, Kone-Paut I, Sarles J, et al. Anakinra is safe and effective in controlling hyperimmunoglobulinaemia D syndrome-associated febrile crisis. J Inherit Metab Dis 2006;29(6):763.
Simon A, Drewe E, van der Meer JW, Powell RJ, Kelley RI, Stalenhoef AF, et al. Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clin Pharmacol Ther 2004;75(5):476–83.
Williamson LM, Hull D, Mehta R, Reeves WG, Robinson BH, Toghill PJ. Familial Hibernian fever. Q J Med 1982;51(204):469–80.
McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999;97(1):133–44.
Aganna E, Hammond L, Hawkins PN, Aldea A, McKee SA, van Amstel HK, et al. Heterogeneity among patients with tumor necrosis factor receptor-associated periodic syndrome phenotypes. Arthritis Rheum 2003;48(9):2632–44.
D’Osualdo A, Ferlito F, Prigione I, Obici L, Meini A, Zulian F, et al. Neutrophils from patients with TNFRSF1A mutations display resistance to tumor necrosis factor-induced apoptosis—pathogenetic and clinical implications. Arthritis Rheum 2006;54(3):998–1008.
Hull KM, Drewe E, Aksentijevich I, Singh HK, Wong K, McDermott EM, et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81(5):349–68.
Kile RL, Rusk HA. A case of cold urticaria with an unusual family history. JAMA 1940;114:1067–8.
Muckle TJ, Well SM. Urticaria, deafness, and amyloidosis: a new heredo-familial syndrome. Q J Med 1962;31:235–48.
Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr 1981;99(1):79–83.
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat Genet 2001;29(3):301–5.
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 2004;20(3):319–25.
Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol 2001;108(4):615–20.
Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med 2007;356(23):2361–71.
Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle–Wells syndrome and response to anakinra. Arthritis Rheum 2004;50(2):607–12.
Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 2006;355(6):581–92.
Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA, et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum 2007;56(9):3138–48.
Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr 1985;107(5):689–93.
Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001;29(1):19–20.
Rose CD, Wouters CH, Meiorin S, Doyle TM, Davey MP, Rosenbaum JT, et al. Pediatric granulomatous arthritis: an international registry. Arthritis Rheum 2006;54(10):3337–44.
Arostegui JI, Arnal C, Merino R, Modesto C, Antonia CM, Moreno P, et al. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum 2007;56(11):3805–13.
Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin Proc 1997;72(7):611–5.
Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet 2002;11(8):961–9.
Majeed HA, Kalaawi M, Mohanty D, Teebi AS, Tunjekar MF, al-Gharbawy F, et al. Congenital dyserythropoietic anemia and chronic recurrent multifocal osteomyelitis in three related children and the association with Sweet syndrome in two siblings. J Pediatr 1989;115(5 Pt 1):730–4.
Majeed HA, Al-Tarawna M, El-Shanti H, Kamel B, Al-Khalaileh F. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. Eur J Pediatr 2001;160(12):705–10.
Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J Med Genet 2005;42(7):551–7.
Ferguson PJ, Bing X, Vasef MA, Ochoa LA, Mahgoub A, Waldschmidt TJ, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone 2006;38(1):41–7.
Marshall GS, Edwards KM, Butler J, Lawton AR. Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J Pediatr 1987;110(1):43–6.
Thomas KT, Feder HM, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr 1999;135(1):15–21.
Gattorno M, Sormani MP, Federici S, Pelagatti L, et al. Validation of a diagnostic score for molecular analysis of hereditary autoinflammatory syndromes in children with periodic fever. Arthritis Rheum 2007;56(9 Suppl):S790.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gattorno, M., Federici, S., Pelagatti, M.A. et al. Diagnosis and Management of Autoinflammatory Diseases in Childhood. J Clin Immunol 28 (Suppl 1), 73–83 (2008). https://doi.org/10.1007/s10875-008-9178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9178-3