Abstract
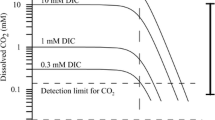
Accurate and rapid determination of inorganic carbon constituents in ocean environments is important for understanding the carbon cycle, especially in the context of ocean-acidification research. A microsensor capable of directly measuring carbonate ion (CO3 2–) concentrations would be desirable. In this study, a carbonate microsensor with a polymeric liquid membrane was fabricated, and two calibration methods were used to evaluate its performance. The first method was based on continuous titration. Small increments of HCl were added to seawater or Na2CO3 solution to adjust the total alkalinity and pH values and thus obtain a series of carbonate concentrations. The second method used a series of discrete standards. Varying amounts of HCl or NaOH were added to separate seawater aliquots, and the CO3 2– concentration of each standard was calculated from the resulting total alkalinity and total dissolved inorganic carbon. Both methods were found to be adequate for achieving accurate calibration of the CO3 2– sensor, and both are suitable for field work. The discrete standards method, however, is more convenient and may provide a better linear range at low CO3 2– concentrations (detection range: 2–300 μmol/kg) than the continuous titration method in seawater (detection range: 10–250 μmol/kg). This CO3 2– microsensor can be used for 5–7 d and detects changes in carbonate concentration as low as 2 μmol/kg in the inorganic carbon constituents of the environments where marine calcareous organisms grow. The CO3 2– microelectrode was further assessed by applying it to the measurement of pore-water CO3 2– concentration profiles in a marine sediment core.






Similar content being viewed by others
References
Alamdari A, Rahimpour MR, Esfandiari N (2008) Kinetics of magnesium hydroxide precipitation from sea bittern. Chem Eng Proc 47(2):215–221
Barker S, Higgins JA, Elderfield H (2003) The future of the carbon cycle: review, calcification response, ballast and feedback on atmospheric CO2. Philos Trans R Soc A 361:1977–1998 (ISBN 1364-503X)
Blaz T, Migdalski J, Lewenstam A (2005) Junction-less reference electrode for potentiometric measurements obtained by buffering pH in a conducting polymer matrix. Analyst 130(5):637–643. doi:10.1039/B418384C
Cai WJ, Reimers CE (1993) The development of pH and pCO2 microelectrodes for studying the carbonate chemistry of pore waters near the sediment–water interface. Limnol Oceanogr 38:1776–1787
Cai WJ, Reimers CE (2000) Sensors for in situ pH and pCO2 measurements in seawater and at the sediment–water interface. In: Buffle J, Horvai G (eds) In-situ monitoring of aquatic system: chemical analysis and speciation. IUPAC Book Series on Analytical and Physical Chemistry of Environmental Systems, vol 6. Wiley, New York
Cai WJ, Wang YC (1998) The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha River, Georgia. Limnol Oceanogr 43(4):657–668
Cai WJ, Zhao PS, Wang YC (2000) pH and pCO2 microelectrode measurements and the diffusive behavior of carbon dioxide species in coastal marine sediments. Mar Chem 70(1–3):133–148. doi:10.1016/S0304-4203(00)00017-7
Cai WJ, George WL, Jeffrey CC, Biblin AE (2010) Carbon cycling and the coupling between proton and electron transfer reactions in aquatic sediments in Lake Champlain. Aqua Geochem 16:421–446. doi:10.1007/s10498-010-9097-9
Choi YS, Lvova L, Shin JH, Oh SH, Lee CS, Kim BH, Cha GS, Nam H (2002) Determination of oceanic carbon dioxide using a carbonate-selective electrode. Anal Chem 74(10):2435–2440. doi:10.1021/ac0108459
de Beer D, Bissett A, de Wit R, Jonkers H, Kohler-Rink S, Nam H, Kim BH, Eickert G, Grinstain M (2008) A microsensor for carbonate ions suitable for microprofiling in freshwater and saline environments. Limnol Oceanogr Methods 6:532–541
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res Part A 34(10):1733–1743
Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ (2004) Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305(5682):362–366
Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Annals NY Acad Sci 1134:320–342. doi:10.1196/annals.1439.013
Herman HB, Rechnitz GA (1975) Preparation and properties of a carbonate ion-selective membrane electrode. Anal Chim Acta 76(1):155–164
Hoegh-Guldberg O (2005) Low coral cover in a high-CO2 world. J Geophys Res 110(9). doi: 10.1029/2004JC002528
Huang WJ, Wang YC, Cai WJ (2012) Assessment of sample storage techniques for total alkalinity and dissolved inorganic carbon in seawater. Limnol Oceanogr-Meth 10:711–717. doi:10.4319/lom.2012.10.711
Jahnke RA, Jahnke DB (2004) Calcium carbonate dissolution in deep sea sediments: reconciling microelectrode, pore water and benthic flux chamber results. Geochim Cosmochim Acta 68(1):47–59
Langdon C (2005) Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res 110(9):1–16
Langdon C, Broecker WS, Hammond DE, Glenn E, Fitzsimmons K, Nelson SG, Peng TH, Hajdas I, Bonani G (2003) Effect of elevated CO2 on the community metabolism of an experimental coral reef. Global Biogeochem Cy 17(1):1–14. doi:10.1029/2002GB001941
Lee HJ, Yoon IJ, Yoo CL, Pyun HJ, Cha GS, Nam H (2000) Potentiometric evaluation of solvent polymeric carbonate-selective membranes based on molecular tweezer-type neutral carriers. Anal Chem 72(19):4694–4699. doi:10.1021/ac9912121
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center (Oak Ridge National Laboratory, US Department of Energy), Oak Ridge
Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RN (1973) Measurement of the apparent dissociation constants of carbonic acid in seawater at atmosphere pressure. Limnol Oceanogr 18(6):897–907
Millero FJ, Lee K, Roche M (1998) Distribution of alkalinity in the surface waters of the major oceans. Mar Chem 60:111–130
Müller B, Buis K, Stierli R, Wehrli B (1998) High spatial resolution measurements in lake sediments with PVC based liquid membrane ion-selective electrodes. Limnol Oceanogr 43(7):1728–1733
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner GK, Rdogers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig MF, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437(29):681–686. doi:10.1038/nature04095
Pyun HJ, Chu J, Yoon W, Jun YM, Kim DJ (1999) Synthesis and evaluation of the cholic acid derivatives with multi-trifluoroacetylbenzoyl (TFAB) groups as carbonate ionophores. Bull Korean Chem Soc 20(2):179–186
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407(6802):364–367
Sabine CL, Feely RA, Key RM, Bullister JL, Millero FJ, Lee K, Peng TH, Tibrook B, Ono T, Wong CS (2002) Distribution of anthropogenic CO2 in the Pacific Ocean. Global Biogeochem Cy 16(4):1–17. doi:10.1029/2001GB001639
Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DW, Tibrook B, Millero FJ, Peng TH, Kozyr A, Ono T, Rios AF (2004) The oceanic sink for anthropogenic CO2. Science 305(5682):367–371
Shaw T (2009) Review of Chemical Oceanography and the Marine Carbon Cycle, by Emerson SR and Hedges JI. Oceanogr 22(2):261–263. doi:10.5670/oceanog.2009.60
Wise WM, Corning NY (1973) Bicarbonate ion sensitive electrode. US Patent 222023
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B 275(1644):1767–1773
Zhao PS, Cai WJ (1997) An improved potentiometric pCO2 microelectrode. Anal Chem 69(24):5052–5058. doi:10.1021/ac970747g
Zhao PS, Cai WJ (1999) pH polymeric membrane microelectrodes based on neutral carriers and their application in aquatic environments. Anal Chim Acta 395(3):285–291
Acknowledgments
This study was supported by the US National Science Foundation (no. EF1041070 to Cai); the Youth Foundation for Marine Science of State Oceanic Administration, Public Research Institutes (Second Institute of Oceanography, State Oceanic Administration, PRC, under contract no. 2013529). We are most grateful to Prof. C. Chen and Dr. X. Hu for helping to review and revise the paper and to Prof. Y. Zhang and Dr. Q. Zhao for providing helpful suggestions. We thank Prof. Nakhyun Nam for providing the carbonate ionophore and Charles Schutte for providing the sediment core. This work was also supported by the China Scholarship Council. Under this sponsorship, the first author (Han) was a visiting student in Cai’s laboratory at the University of Georgia while this work was accomplished.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, C., Cai, WJ., Wang, Y. et al. Calibration and evaluation of a carbonate microsensor for studies of the marine inorganic carbon system. J Oceanogr 70, 425–433 (2014). https://doi.org/10.1007/s10872-014-0243-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-014-0243-7