Abstract
The polymorphism of the glycoside donor methyl 2,3,4-tri-O-acetyl-1-O-(trichloroacetimidoyl)-α-d-glucopyranouronate (1) has been investigated. Two polymorphic forms (labelled Forms I and II) have been elucidated and fully characterised by DSC, PXRD and single crystal analysis, both crystallizing in the space group P21. Form I was obtained by crystallization from a wide range of solvents, while Form II was obtained only from ethyl acetate or isopropanol on certain occasions. Unit cell dimensions for Form I are a 14.0292(12), b 8.9641(8), c 16.8580(14) Å, β 94.285(2)°, and for Form II a 11.266(3), b 6.8889(17), c 13.921(4) Å, β 101.161(6)°. Z’ is 2 for Form I and 1 for Form II. Form I displays two moderate intermolecular hydrogen bonds in the unit cell whereas Form II shows no moderate hydrogen-bonding motifs. All three molecules in the two polymorphs differ significantly in their conformations, especially with respect to the methyl carboxylate and trichloroacetimidoyl groups.
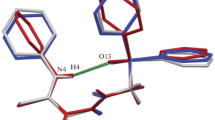
Graphical Abstract
Structures of two polymorphs of methyl 2,3,4-tri-O-acetyl-1-O-(trichloroacetimidoyl)-α-d-glucopyranouronate were determined.







Similar content being viewed by others

References
Bernstein J (2002) Polymorphism in Molecular Crystals. Oxford Science Publications, Oxford
Braga D, Grepioni F, Maini L (2010) Chem Commun 46:6232
Bernstein J, Hagler AT (1978) J Am Chem Soc 100:673
Mukuta T, Lee AY, Kawakami T, Myerson AS (2005) Cryst Growth Des 5:1429
Schmidt RR (1986) Angew Chem Int Ed Engl 25:212
Fischer B, Nudelman A, Ruse M, Herzig J, Gottlieb HE (1984) J Org Chem 49:4988
Stachulski AV, Jenkins G (1998) Nat Prod Rep 15:173
Brown RT, Carter NE, Mayalarp SP, Scheinmann F (2000) Tetrahedron 56:7591
Kuszmann J, Medgyes G, Boros S (2004) Carbohydr Res 339:1569
Hayes JA, Eccles KS, Lawrence SE, Moynihan HA (2012) Carbohydr Res 349:108
Bruker AXS (2009) APEX2 v2009.3–0. Madison, WI
Sheldrick GM (2008) Acta Crystallogr A 64:112
Macrae CF, Bruno IJ, Chrisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) J Appl Crystallogr 41:466
Spek AL (2009) Acta Cryst D65:148
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354
Flack HD (1983) Acta Crystallogr A39:876
Cai C, Wei G, Du Y (2010) Acta Cryst E66:o949
Baddeley TC, Howie RA, Skakle JMS, Wardell JL (2005) Acta Cryst C61:o711
Acknowledgments
This publication has emanated from research conducted with the financial support of Science Foundation Ireland under Grant Numbers 07/SRC/B1158, 08/RFP/MTR1664 and 05/PICA/B802/EC07.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayes, J.A., Eccles, K.S., Elcoate, C.J. et al. Crystal Polymorphism of Methyl 2,3,4-tri-O-acetyl-1-O-(trichloroacetimidoyl)-α-d-glucopyranouronate. J Chem Crystallogr 43, 138–143 (2013). https://doi.org/10.1007/s10870-013-0397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-013-0397-y