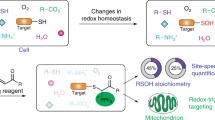
Abstract
Studies on chemical modifications of bacterial and mitochondrial complex I by synthetic chemical probes as well as endogenous chemicals have provided useful information on the structural and functional aspects of this enzyme. We herein reviewed recent studies that investigated chemical modifications of complex I by endogenous chemicals (e.g. Cys-S-nitrosation, Cys-S-glutathionylation, and Ser-O-phosphorylation) and synthetic reagents (e.g. Cys-SH modification by SH-reagents and the cross-linking of nearby subunits by bifunctional cross-linkers). We also reviewed recent photoaffinity labeling studies using complex I inhibitors, which can be recognized as “site-specific modification” by synthetic chemicals. In addition, we discussed the possibility of site-specific modification by various functional probes via ligand-directed tosylate (LDT) chemistry as a promising approach for unique biophysical studies on complex I.

Similar content being viewed by others

References
Andrews B, Carroll J, Ding S, Feanley IM, Walker JE (2014) Assembly factors for the membrane arm of human complex I. Proc Natl Acad Sci U S A 110(47):18934–9
Antos JM, McFarland JM, Lavarone AT, Francis MB (2009) Chemoselective tryptophan labeling with rhodium carbenoids at mild pH. J Am Chem Soc 131(17):6301–6308
Babot M, Labarbuta P, Birch A, Fuszard M. Botting GH, Wittig I, Heide H, Galkin A (2014) ND3, ND1 and 39 kDa subunits are more exposed in the deactive form of bovine mitochondrial complex I, Biochim. Biophys. Acta 1837 (6):929–939
Baradaran R, Berrisford JM, Minhas GS, Sazanov LA (2013) Crystal structure of the entire respiratory complex I. Nature 494(7438):443–448
Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP (2004) Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins. J Biol Chem 279(46):47939–47591
Belogrudov G, Hatefi Y (1994) Catalytic sector of complex I (NADH:ubiquinone oxidoreductase): subunit stoichiometry and substrate-induced conformation changes. Biochemistry 33(15):4571–4576
Bernardes GJL, Chalker JM, Errey JC, Davis BG (2008) Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J Am Chem Soc 130(15):5052–5053
Berrisford JM, Thompson CJ, Sazanov LA (2008) Chemical and NADH-induced, ROS-dependent, cross-linking between subunits of complex I from Escherichia coli and Thermus thermophilus. Biochemistry 47(39):10262–10270
Brandt U (2006) Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem 75:69–92
Cai K, Itoh Y, Khorane HG (2001) Mapping of contact sites in complex formation between transducing and light-activated rhodopsin by covalent crosslinking: use of a photoactivatable reagent. Proc Natl Acad Sci U S A 98(9):4877–4882
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria. J Biol Chem 278(38):36027–36031
Chen R, Fearnley IM, Peak-Chew SY, Walker JE (2004) The phosphorylation of subunits of complex I from bovine heart mitochondria. J Biol Chem 279(25):26036–26045
Chouchani ET, Hurd TR, Nadtochiy SM, Brookes PS, Fearnley IM, Lilley KS, Smith RAJ, Murphy MP (2010) Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J 430(1):49–59
Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cochemé HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RAJ, Krieg T, Brookes PS, Murphy MP (2013) Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19(6):753–759
Ciano M, Fuszard M, Heide H, Botting GH, Galkin A (2013) Conformation-specific crosslinking of mitochondrial complex I. FEBS Lett 587(7):867–872
Di Bernardo S, Yagi T (2001) Direct interaction between a membrane domain subunit and a connector subunit in the H+-translocating NADH-quinone oxidoreductase. FEBS Lett 508(3):385–388
Dorman G, Prestwich GD (2000) Using photolabile ligands in drug discovery and development. Trends Biotechnol 18(2):64–77
Dröse S, Brandt U, Wittig I (2014) Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 1844(8):1344–1354
Efremov RG, Sazanov LA (2012) The coupling mechanism of respiratory complex I: A structural and evolutionary perspective. Biochim Biophys Acta 1817(10):1785–1795
Efremov RG, Baradaran R, Sazanov LA (2010) The architecture of respiratory complex I. Nature 465(7297):441–445
Galkin A, Moncada S (2007) S-Nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem 282(52):37448–37453
Galkin A, Meyer B, Wittig I, Karas M, Schägger H, Vinogradov A, Brandt U (2008) Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J Biol Chem 283(30):20907–20913
Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S (2009) Lack of oxygen deactivates mitochondrial complex I. J Biol Chem 284(52):36055–36061
Gallogy MM, Mieyal JJ (2007) Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr, Opin. Pharmacol 7(4):381–391
Gavrikova EV, Vinogradov AD (1999) Active-deactive state transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Lett 455(1–2):36–40
Gondal JA, Anderson WM (1985) The molecular morphology of bovine heart mitochondrial NADH-ubiquinone reductase. Native disulfide-linked subunits and rotenone-induced conformational changes. J Biol Chem 260(23):12690–12694
Gostimskaya IS, Cecchini G, Vinogradov AD (2006) Topography and chemical reactivity of the active-inactive transition-sensitive SH-group in the mitochondrial NADH:ubiquinone oxidoreductase (complex I). Biochim Biophys Acta 1757(9–10):21155–1161
Grivennikova VG, Kapustin AN, Vinogradov AD (2001) Catalytic activity of NADH-ubiquinone oxidoreductase (complex I) in intact mitochondria. J Biol Chem 276(12):9038–9044
Hatanaka Y, Sadakane Y (2002) Photoaffinity labeling in drug discovery and developments: Chemical gateway for entering proteomic frontier. Curr Top Med Chem 2(3):271–288
Hirst J (2010) Towards the molecular mechanism of respiratory complex I. Biochem J 425(2):327–339
Hirst J (2013) Mitochondrial complex I. Annu Rev Biochem 82:551–575
Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP (2007) Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis. J Biol Chem 282(30):22040–22051
Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP (2008) Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys704 of the 75 kDa subunit. J Biol Chem 283(36):24801–24815
Kakutani N, Murai M, Sakiyama N, Miyoshi H (2010) Exploring the binding site of Δlac-acetogenin in bovine heart mitochondrial NADH-ubiquinone oxidoreductase. Biochemistry 49(23):4794–4803
Kao MC, Matsuno-Yagi A, Yagi T (2004) Subunit proximity in the H+-translocating NADH-quinone oxidoreductase probed by zero-length cross-linking. Biochemistry 43(12):3750–3755
Kmita K, Zickermann V (2013) Accessory subunits of mitochondrial complex I. Biochem Soc Trans 41(5):1272–1279
Kumar V, Calamaras TD, Haeussler D, Colucci WS, Cohen RA, Mccomb ME, Pimentel D, Bachschmid MM (2012) Cardiovascular redox and ox stress proteomics, Antioxid. Redox Signal 17(11):1528–1559
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain, J. J Neurochem 80(5):780–787
Loewen MC, Klein-Seetharaman J, Getmanova EV, Reeves PJ, Schwalbe H, Khorane HG (2001) Solution 19 F nuclear Overhauser effects in structural studies of the cytoplasmic domain of mammalian rhodopsin. Proc Natl Acad Sci U S A 98(9):4888–4892
Mailloux RJ, Seifert EL, Bouillaud F, Auger C, Collins S, Harper M-E (2011) Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 286(24):21865–21875
Mailloux RJ, Jin X, Willmore WG (2014) Redox regulation of mitochondrial function with emphasis on cystein oxidation reactions. Redox Biol 2:123–139
Mamedova AA, Holt PJ, Carroll J, Sazanov LA (2004) Substrate-induced conformational change in bacterial complex I. J Biol Chem 279(22):23830–23836
Masuya T, Murai M, Ifuku K, Morisaka H, Miyoshi H (2014) Site-specific chemical labeling of mitochondrial respiratory complex I through ligand-directed tosylate chemistry. Biochemistry 53(14):2307–2317
McFarland JM, Francis MB (2005) Reductive alkylation of proteins using iridium catalyzed transfer hydrogenation. J Am Chem Soc 127(39):13490–13491
Minakami S, Schindler FJ, Estabrook RW (1964a) Hydrogen transfer between reduced diphosphopyridine nucleotide dehydrogenase and respiratory chain: I effect of sulfhydryl inhibitors and phospholipase. J Biol Chem 239:2042–2048
Minakami S, Schindler FJ, Estabrook RW (1964b) Hydrogen transfer between reduced diphosphopyridine nucleotide dehydrogenase and the respiratory chain: II an initial lag in the oxidation of reduced diphosphopyridine nucleotide J. Biol Chem 239:2049–2054
Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grünewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B (2014) PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science 344(6180):203–207
Murai M, Ishihara A, Nishioka T, Yagi T, Miyoshi H (2007) The ND1 subunit constructs the inhibitor binding domain in bovine heart mitochondrial complex I. Biochemistry 46(21):6409–6416
Murai M, Sekiguchi K, Nishioka T, Miyoshi H (2009) Characterization of the inhibitor binding site in mitochondrial NADH-ubiquinone oxidoreductase by photoaffinity labeling using a quinazoline-type inhibitor. Biochemistry 48(4):688–698
Murai M, Mashimo Y, Hirst J, Miyoshi H (2011) Exploring interactions between the 49 kDa and ND1 subunits in mitochondrial NADH-ubiquinone oxidoreductase (complex I) by photoaffinity labeling. Biochemistry 50(32):6901–6908
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13
Murphy MP (2012) Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications, Antioxid. Redox Signal 16(6):477–495
Murray CI, Kane LA, Uhrigshardt H, Wang SB, Van Eyk JE (2011) Site-mapping of in vitro S-nitrosation in cardiac mitochondria: implications for cardioprotection. Mol. Cell Prot. 10 (3): M110.004721.
Nakanishi S, Abe M, Yamamoto S, Murai M, Miyoshi H (2011) Bis-THF motif of acetogenin binds to the third matrix-side loop of ND1 subunit in mitochondrial NADH-ubiquinone oxidoreductase. Biochim Biophys Acta 1807(9):1170–1176
Papa S, Sardanelli AM, Cocco T, Speranza F, Scacco SC, Technikova-Dobrova Z (1996) The nuclear-encoded 18 kDa (IP) AQDQ subunit of bovine heart complex I is phosphorylated by the mitochondrial cAMP-dependent protein kinase. FEBS Lett 379(3):299–301
Pawson T (2007) Dynamic control of signaling by modular adaptor proteins. Curr Opin Cell Biol 19(2):112–116
Pohl T, Uhlmann M, Kaufenstein M, Friedrich T (2007) Lambda red-mediated mutagenesis and efficient large scale affinity purification of the Escherichia coli NADH:ubiquinone oxidoreductase (complex I). Biochemistry 46(37):10694–10702
Pohl T, Spatzal T, Aksoyoglu M, Rostas AM, Lay H, Glessner U, Boudon C, Hellwig P, Weber S, Friedrich T (2010) Spin labeling of the Escherichia coli NADH ubiquinone oxidoreductase (complex I). Biochim Biophys Acta 1797(12):1894–1900
Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RAJ, Murphy MP (2009) A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A 106(26):10764–10769
Pryde KR, Hirst J (2011) Superoxide is produced by the reduced flavin in mitochondrial complex I. J Biol Chem 286(20):18056–18065
Robert PG, Hirst J (2012) The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter. J Biol Chem 287(41):34743–34751
Sanada S, Komuro I, Kitakaze M (2011) Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 301(5):H1723–H1741
Sazanov LA, Hinchliffe P (2006) Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311(5766):1430–1436
Schilling B, Aggeler R, Schulenberg B, Murray J, Row RH, Capaldi RA, Gibson BW (2005) Mass spectrometric identification of a novel phosphorylation site in subunit NDUFA10 of bovine mitochondrial complex I. FEBS Lett 579(11):2485–2490
Schlick TL, Ding Z, Kovacs EW, Francis MB (2005) Dual-surface modification of the tobacco mosaic virus. J Am Chem Soc 127(11):3718–3723
Schulenberg B, Aggeler R, Beechem JM, Capaldi RA, Patton WF (2003) Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. J Biol Chem 278(29):27251–27255
Sekiguchi K, Murai M, Miyoshi H (2009) Exploring the binding site of acetogenin in the ND1 subunit of bovine mitochondrial complex I. Biochim Biophys Acta 1787(9):1106–1111
Shiraishi Y, Murai M, Sakiyama N, Ifuku K, Miyoshi H (2012) Fenpyroximate binds to the interface between PSST and 49 kDa subunits in mitochondrial NADH-ubiquinone oxidoreductase. Biochemistry 51(9):1953–1963
Smith RAJ, Porteous CM, Gane AM, Murphy MP (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A 100(9):5407–5412
Stephanopoulos N, Francis MB (2011) Choosing an effective protein bioconjugation strategy, Nat. Chem Biol 7(11):876–884
Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E (2007) Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101(11):1155–1163
Takaoka Y, Ojida A, Hamachi I (2013) Protein organic chemistry and applications for labeling and engineering in live-cell systems. Angew Chem Int Ed 52(15):4088–4106
Taylor ER, Hurrell F, Shannon RJ, Lin T-K, Hirst J, Murphy MP (2003) Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem 278(22):19603–19610
Tocilescu MA, Zickermann V, Zwicker K, Brandt U (2010) Quinone binding and reduction by respiratory complex I, Biochim. Biophys. Acta 1797 (12):18831890.
Toda N, Asano S, Barbas CF III (2014) Rapid, stable, chemoselective labeling of thiols with Julia-Kocienski-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew Chem Int Ed 52(48):12592–12596
Tsukiji S, Miyagawa M, Takaoka Y, Tamura T, Hamachi I (2009) Ligand-directed tosyl chemistry for protein labeling in vivo, Nat. Chem Biol 5(5):341–343
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674):1158–1160
Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG (2003) Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc 125(11):3192–3193
Watanabe R, Tabata KV, Iino R, Ueno H, Iwamoto M, Oiki S, Noji H (2013) Biased Brownian stepping rotation of FoF1-ATP synthase driven by proton motive force. Nat Comm 4:1631
Yamaguchi M, Hatefi Y (1993) Mitochondrial NADH:ubiquinone oxidoreductase (complex I): proximity of the subunits of the flavoprotein and the iron − sulfur protein subcomplexes. Biochemistry 32(8):1935–1939
Yasuda R, Masaike T, Adachi K, Noji H, Itoh H, Kinosita K Jr (2003) The ATP-waiting conformation of rotating F1-ATPase revealed by single-pair fluorescence resonance energy transfer. Proc Natl Acad Sci U S A 100(16):9314–9318
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (Grant 23380064 to H. M.) and for Young Scientists (Grant 23780116 to M. M.) from the Japan Society for the Promotion of Science.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murai, M., Miyoshi, H. Chemical modifications of respiratory complex I for structural and functional studies. J Bioenerg Biomembr 46, 313–321 (2014). https://doi.org/10.1007/s10863-014-9562-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-014-9562-z