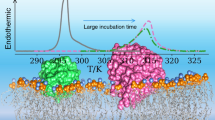
Abstract
In this paper, we report on the effect of short segments of type I antifreeze protein (AFP I) on the thermotropic properties of a model membrane. Two different types of dimyristoylphosphatidylcholine model membranes were used, multilamellar vesicles and small unilamellar vesicles. The membrane properties were studied by differential scanning calorimetry (DSC) and fluorescence anisotropy. With the incorporation of AFP I and its short segments, the order of the model membrane increased both in the gel state and in the liquid crystalline state. The interaction of AFPs with the model membrane caused a shift in the phase transition to lower temperatures, which is accompanied by a broadening of the DSC thermogram. This preferential stabilization to a more ordered phase by the AFPs could be due to ordering the hydrophobic membrane core and separation into domains. Overall, this approach of employing short segments of AFP I simplifies the correlation between antifreeze protein characteristics and the effect of these parameters on the interaction mechanism of AFP with cell membranes. The success of this approach can lead to the identification of short peptides with high antifreeze activity.
Similar content being viewed by others
References
Adler M, Tritton TR (1988) Fluorescence depolarization measurements on oriented membranes. Biophys J 53:989–1005
Andrich MP, Vanderkooi JM (1976) Temperature-dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phospholipid artificial membranes. Biochemistry 15:1257–1261
Barrett J (2001) Thermal hysteresis proteins. Int J Biochem Cell B 33:105–117
Blandamer MJ, Briggs B, Cullis PM, Engberts JBFN (1995) Gel to liquid-crystal transitions in synthetic amphiphile vesicles. Chem Soc Rev 24:251
Borenstain V, Barenholz Y (1993) Characterization of liposomes and other lipid assemblies by multiprobe fluorescence polarization. Chem Phys Lipids 117–127:64
Bouvet V, Ben RN (2003) Antifreeze glycoproteins—structure, conformation, and biological applications. Cell Biochem Biophys 39:133–144
Crowe JH, Tablin F, Tsvetkova N, Oliver AE, Walker N, Crowe LM (1999) Are lipid phase transitions responsible for chilling damage in human platelets? Cryobiology 38:180–191
Davies PL, Hew CL (1990) Biochemistry of fish antifreeze proteins. Faseb J 4:2460–2468
Denich TJ, Beaudette LA, Lee H, Trevors JT (2003) Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Meth 52:149–182
Devries AL, Wohlschl DE (1969) Freezing resistance in some antarctic fishes. Science 163:1073
Devries AL, Vandenhe J, Feeney RE (1971) Peimary structure of freezing point-depressing glycoproteins. J Biol Chem 246:305
Duman JG, Wu DW, Xu L, Tursman D, Olsen TM (1991) Adaptations of insects to subzero temperatures. Quart Rev Biol 66:387–410
Feeney RE, Burcham TS, Yeh Y (1986) Antifreeze glycoproteins from polar fish blood. Annu Rev Biophys Bio 15:59–78
Feller G, Gerday C (1997) Psychrophilic enzymes: molecular basis of cold adaptation. Cell Mol Life Sci 53:830–841
Fletcher GL, Hew CL, Davies PL (2001) Antifreeze proteins of teleost fishes. Annu Rev Physiol 63:359–390
Fuchs P, Parola A, Robbins PW, Blout ER (1975) Fluorescence polarization and viscosities of membrane lipids of 3t3 cells. P Natl Acad Sci U S A 72:3351–3354
Hays LM, Feeney RE, Crowe LM, Crowe JH, Oliver AE (1996) Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. P Natl Acad Sci U S A 93:6835–6840
Hays LM, Crowe JH, Wolkers W, Rudenko S (2001) Factors affecting leakage of trapped solutes from phospholipid vesicles during thermotropic phase transitions. Cryobiology 42:88–102
Hincha DK, Devries AL, Schmitt JM (1993) Cryotoxicity of antifreeze proteins and glycoproteins to spinach thylakoid membranes - comparison with cryotoxic sugar acids. Biochim Biophys Acta 1146:258–264
Kaiser RD, London E (1998) Location of diphenylhexatriene (DPH) and its derivatives within membranes: Comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 37:8180–8190
Komatsu SK, Devries AL, Feeney RE (1970) Studies of structure of freezing point-depressing glycoproteins from an antarctic fish. J Biol Chem 245:2909
Kun H, Mastai Y (2007) Activity of short segments of type I antifreeze protein. Biopolymers 88:807–814
Mabrey S, Mateo PL, Sturtevant JM (1978) High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoylphosphatidylcholines and dipalmitoylphosphatidylcholines. Biochemistry 17:2464–2468
Marsh D (1990) Handbook of lipids bilayers. CRC, Boca Raton, Florida
Negulescu PA, Rubinsky B, Fletcher GL, Machen TE (1992) Fish antifreeze proteins block Ca entry into rabbit parietal cells. Am J Physiol Cell Physiol 263:C1310–C1313
Ricker JV, Tsvetkova NM, Wolkers WF, Leidy C, Tablin F, Longo M, Crowe JH (2003) Trehalose maintains phase separation in an air-dried binary lipid mixture. Biophys J 84:3045–3051
Rubinsky B, Arav A, Mattioli M, Fletcher GL (1990) The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem Biophys Res Comm 173:1369–1374
Rubinsky B, Mattioli M, Arav A, Barboni B, Fletcher GL (1992) Inhibition of Ca2+ and K+ currents by antifreeze proteins. Am J Physiol 262:R542–R545
Somero GN, Devries AL (1967) Temperature tolerance of some antarctic fishes. Science 156:257
Tablin F, Oliver AE, Walker NJ, Crowe LM, Crowe JH (1996) Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J Cell Physiol 168:305–313
Tomczak MM, Crowe JH (2002) The interaction of antifreeze proteins with model membranes and cells. In Fish Antifreeze Proteins World Scientific Publ., UK
Tomczak MM, Hincha DK, Estrada SD, Feeney RE, Crowe JH (2001) Antifreeze proteins differentially affect model membranes during freezing. Bba-Biomembranes 1511:255–263
Tomczak MM, Hincha DK, Estrada SD, Wolkers WF, Crowe LM, Feeney RE, Tablin F, Crowe JH (2002a) A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys J 874–881:882
Tomczak MM, Vigh L, Meyer JD, Manning MC, Hincha DK, Crowe JH (2002b) Lipid unsaturation determines the interaction of AFP type I with model membranes during thermotropic phase transitions. Cryobiology 45:135–142
Tomczak MM, Hincha DK, Crowe JH, Harding MM, Haymet ADJ (2003) The effect of hydrophobic analogues of the type I winter flounder antifreeze protein on lipid bilayers. Febs Lett 551:13–19
Trevors JT (2003) Fluorescent probes for bacterial cytoplasmic membrane research. J Biochem Bioph Meth 57:87–103
Shinitzk M, Inbar M (1974) Difference in microviscosity induced by different cholesterol levels in surface membrane lipid layer of normal lymphocytes and malignant lymphoma-cells. J Mol Biol 85:603–615
Shinitzky M, Barenholz Y (1978) Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 515:367–394
Sklar LA (1984) Fluorescence polarization studies of membrane fluidity, where do we go from here. Plenum, New York
vanderHeide UA, vanGinkel G, Levine YK (1996) DPH is localised in two distinct populations in lipid vesicles. Chem Phys Lett 253:118–122
Wu YL, Fletcher GL (2000) Efficacy of antifreeze protein types in protecting liposome membrane integrity depends on phospholipid class. Bba-Gen Subjects 1524:11–16
Yeh Y, Feeney RE (1978) Anomalous depression of freezing temperature in a biological system. Accounts Chem Res 11:129–135
Yeh Y, Feeney RE (1996) Antifreeze proteins: structures and mechanisms of function. Chem Rev 96:601–617
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kun, H., Minnes, R. & Mastai, Y. Effects antifreeze peptides on the thermotropic properties of a model membrane. J Bioenerg Biomembr 40, 389–396 (2008). https://doi.org/10.1007/s10863-008-9164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-008-9164-8