Abstract
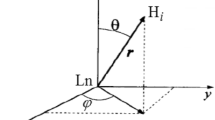
Lanthanide complexes based on the DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) cage are commonly used as phase contrast agents in magnetic resonance imaging, but can also be utilized in structural NMR applications due to their ability to induce either paramagnetic relaxation enhancement or a pseudocontact shift (PCS) depending on the choice of the lanthanide. The size and sign of the PCS for any given atom is determined by its coordinates relative to the metal center, and the characteristics of the lanthanide’s magnetic susceptibility tensor. Using a polymethylated DOTA tag (Ln-M8-SPy) conjugated to ubiquitin, we calculated the position of the metal center and characterized the susceptibility tensor for a number of lanthanides (dysprosium, thulium, and ytterbium) under a range of pH and temperature conditions. We found that there was a difference in temperature sensitivity for each of the complexes studied, which depended on the size of the lanthanide ion as well as the isomeric state of the cage. Using 17O-NMR, we confirmed that the temperature sensitivity of the compounds was enhanced by the presence of an apically bound water molecule. Since amide-containing lanthanide complexes are known to be pH sensitive and can be used as probes of physiological pH, we also investigated the effect of pH on the Ln-M8-SPy susceptibility tensor, but we found that the changes in this pH range (5.0–7.4) were not significant.





Similar content being viewed by others
References
Aime S, Botta M, Ermondi G (1992) NMR study of solution structures and dynamics of lanthanide(III) complexes of DOTA. Inorg Chem 31:4291–4299. doi:10.1021/ic00047a016
Aime S, Botta M, Ermondi G, Terreno E, Anelli PL, Fedeli F, Uggeri F (1996) Relaxometric, structural, and dynamic NMR studies of DOTA-like Ln(III) complexes (Ln = La, Gd, Ho, Yb) containing a p-nitrophenyl substituent. Inorg Chem 35:2726–2736. doi:10.1021/ic950981u
Aime S, Barge A, Botta M, Parker D, de Sousa AS (1997) Prototropic vs whole water exchange contributions to the solvent relaxation enhancement in the aqueous solution of a cationic Gd3+ macrocyclic complex. J Am Chem Soc 119:4767–4768. doi:10.1021/ja963743m
Aime S, Barge A, Botta M, De Sousa AS, Parker D (1998) Direct NMR spectroscopic observation of a lanthanide-coordinated water molecule whose exchange rate is dependent on the conformation of the complexes. Angew Chem Int Ed Engl 37:2673–2675. doi:10.1002/(SICI)1521-3773(19981016)37:19<2673:AID-ANIE2673>3.0.CO;2-%23
Aime S et al (1999) NMR, relaxometric, and structural studies of the hydration and exchange dynamics of cationic lanthanide complexes of macrocyclic tetraamide ligands. J Am Chem Soc 121:5762–5771. doi:10.1021/ja990225d
Aime S, Barge A, delli Castelli D, Fedeli F, Mortillaro A, Nielsen FU, Terreno E (2002) Paramagnetic lanthanide(III) complexes as pH-sensitive chemical exchange saturation transfer (CEST) contrast agents for MRI applications. Magn Reson Med 47:639–648. doi:10.1002/mrm.10106
Aime S, delli Castelli D, Crich SG, Gianolio E, Terreno E (2009) Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc Chem Res 42:822–831. doi:10.1021/ar800192p
Banci L, Bertini I, Cavallaro G, Giachetti A, Luchinat C, Parigi G (2004) Paramagnetism-based restraints for Xplor-NIH. J Biomol NMR 28:249–261. doi:10.1023/B:JNMR.0000013703.30623.f7
Baranyai Z, Brücher T, Iványi T, Király R, Lázár I, Zékány L (2005) Complexation properties of N,N’,N”,N”‘-[1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayltetraki(1-oxoethane-2-1-diyl)]tetrakis[glycine](H4dotagl). Equilibrium, kinetic, and relaxation behavior of the lanthanide(III) complexes. Helv Chim Acta 88:604–617. doi:10.1002/hlca.200590042
Beeby A et al (1999) Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states. J Chem Soc Perk Trans 2:493–503. doi:10.1039/a808692c
Benetollo F, Bombieri G, Calabi L, Aime S, Botta M (2003) Structural variations across the lanthanide series of macrocyclic DOTA complexes: insights into the design of contrast agents for magnetic resonance imaging. Inorg Chem 42:148–157. doi:10.1021/ic025790n
Bertini I, Luchinat C (1999) New applications of paramagnetic NMR in chemical biology. Curr Opin Chem Biol 3:145–151. doi:10.1016/S1367-5931(99)80026-X
Bertini I, Janik MBL, Lee YM, Luchinat C, Rosato A (2001) Magnetic susceptibility tenser anisotropies for a lanthanide ion series in a fixed protein matrix. J Am Chem Soc 123:4181–4188. doi:10.1021/ja0028626
Bleaney B (1972) Nuclear magnetic-resonance shifts in solution due to lanthanide ions. J Magn Reson 8:91–100. doi:10.1016/0022-2364(72)90027-3
Cacheris WP, Nickle SK, Sherry AD (1987) Thermodynamic study of lanthanide complexes of 1,4,7-triazacyclononane-N,N’,N’’-triacetic acid and 1,4,7,10-tetraazacyclododecane-N,N’,N”,N”‘-tetraaceticacid. Inorg Chem 26:958–960. doi:10.1021/ic00253a038
Cacheris WP, Quay SC, Rocklage SM (1990) The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging 8:467–481. doi:10.1016/0730-725X(90)90055-7
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB (1999) Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 99:2293–2352. doi:10.1021/cr980440x
Cornilescu G, Marquardt JL, Ottiger M, Bax A (1998) Validation of protein structure from anisotropic carbonyl chemical shifts in a dilute liquid crystalline phase. J Am Chem Soc 120:6836–6837. doi:10.1021/Ja9812610
de Boer JWM, Sakkers PJD, Hilbers CW, de Boer E (1977) Lanthanide shift-reagents. II. Shift mechanisms. J Magn Reson 25:455–476. doi:10.1016/0022-2364(77)90209-8
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. doi:10.1007/BF00197809
Djanashvili K, Peters JA (2007) How to determine the number of inner-sphere water molecules in lanthanide(III) complexes by 17O NMR spectroscopy. A technical note. Contrast Media Mol Imaging 2:67–71. doi:10.1002/cmmi.132
Graham B et al (2011) DOTA-amide lanthanide tag for reliable generation of pseudocontact shifts in protein NMR spectra. Bioconjug Chem 22:2118–2125. doi:10.1021/bc200353c
Häussinger D, Huang JR, Grzesiek S (2009) DOTA-M8: an extremely rigid, high-affinity lanthanide chelating tag for PCS NMR spectroscopy. J Am Chem Soc 131:14761–14767. doi:10.1021/ja903233w
Hoeft S, Roth K (1993) Struktur und dynamik von lanthanoid-tetraazacyclododecantetraacetat-(DOTA-)komplexen in Lösung. Chem Ber 126:869–873. doi:10.1002/cber.19931260404
Horrocks WD, Sudnick DR (1979) Lanthanide ion probes of structure in biology—laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water-molecules. J Am Chem Soc 101:334–340. doi:10.1021/ja00496a010
Huang Y, Coman D, Ali MM, Hyder F (2015) Lanthanide ion (III) complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraaminophosphonate for dual biosensing of pH with chemical exchange saturation transfer (CEST) and biosensor imaging of redundant deviation in shifts (BIRDS). Contrast Media Mol Imaging 10:51–58. doi:10.1002/cmmi.1604
Jacques V, Desreux JF (1994) Quantitative two-dimensional EXSY spectroscopy and dynamic behavior of a paramagnetic lanthanide macrocyclic chelate: YbDOTA (DOTA=1,4,7,10-tetraazacyclododecane-N,N’,N”,N”‘-tetraacetic acid. Inorg Chem 33:4048–4053. doi:10.1021/ic00096a033
John M, Park AY, Pintacuda G, Dixon NE, Otting G (2005) Weak alignment of paramagnetic proteins warrants correction for residual CSA effects in measurements of pseudocontact shifts. J Am Chem Soc 127:17190–17191. doi:10.1021/ja0564259
Keizers PH, Desreux JF, Overhand M, Ubbink M (2007) Increased paramagnetic effect of a lanthanide protein probe by two-point attachment. J Am Chem Soc 129:9292–9293. doi:10.1021/ja0725201
Krchová T, Kotek J, Jirák D, Havličková J, Císařová I, Hermann P (2013) Lanthanide(III) complexes of aminoethyl-DO3A as PARACEST contrast agents based on decoordination of the weakly bound amino group. Dalton Trans 42:15735–15747. doi:10.1039/c3dt52031e
Krchová T, Gálisová A, Jirák D, Hermann P, Kotek J (2016) Ln(III)-complexes of a DOTA analogue with an ethylenediamine pendant arm as pH-responsive PARACEST contrast agents. Dalton Trans 45:3486–3496. doi:10.1039/c5dt04443j
Kumar K, Chang CA, Tweedle MF (1993) Equilibrium and kinetic studies of lanthanide complexes of macrocyclic polyamino carboxylates. Inorg Chem 32:587–593. doi:10.1021/ic00057a017
Lee MD et al (2015) Compact, hydrophilic, lanthanide-binding tags for paramagnetic NMR spectroscopy. Chem Sci 6:2614–2624. doi:10.1039/c4sc03892d
Lee MD, Dennis ML, Swarbrick JD, Graham B (2016) Enantiomeric two-armed lanthanide-binding tags for complementary effects in paramagnetic NMR spectroscopy. Chem Commun (Camb) 52:7597–7954. doi:10.1039/c6cc02325h
Liu WM et al (2012) A pH-sensitive, colorful, lanthanide-chelating paramagnetic NMR probe. J Am Chem Soc 134:17306–17313. doi:10.1021/ja307824e
Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ (2012) Analytical methods for characterizing magnetic resonance probes. Anal Chem 84:6278–6287. doi:10.1021/ac300527z
McGarvey BR (1979) Temperature-dependence of the pseudo-contact shift in lanthanide shift-reagents. J Magn Reson 33:445–455. doi:10.1016/0022-2364(79)90261-0
Meyer M, Dahaoui-Gindrey V, Lecomte C, Guilard L (1998) Conformations and coordination schemes of carboxylate and carbamoyl derivatives of the tetraazamacrocycles cyclen and cyclam and the relation to their protonation states. Coord Chem Rev 178:1313–1405. doi:10.1016/S0010-8545(98)00169-6
Mittag T, Forman-Kay JD (2007) Atomic-level characterization of disordered protein ensembles. Curr Opin Struct Biol 17:3–14. doi:10.1016/j.sbi.2007.01.009
Müntener T, Häussinger D, Selenko P, Theillet FX (2016) In-cell protein structures from 2D NMR experiments. J Phys Chem Lett 7:2821–2825. doi:10.1021/acs.jpclett.6b01074
Opina ACL, Wu Y, Zhao P, Kiefer G, Sherry AD (2011) The pH sensitivity of –NH exchange in LnDOTA-tetraamide complexes varies with amide substituent. Contrast Media Mol Imaging 6:459–464. doi:10.1002/cmmi.445
Opina AC, Strickland M, Lee YS, Tjandra N, Byrd AR, Swenson RE, Vasalatiy O (2016) Analysis of the isomer ratios of polymethylated-DOTA complexes and the implications on protein structural studies. Dalton Trans 45:4673–4687. doi:10.1039/c5dt03210e
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi:10.1002/jcc.20084
Pinkerton AA, Rossier M, Spiliadis S (1985) Lanthanide-induced contact shifts—the average electron-spin polarization. Theory Exp J Magn Reson 64:420–425. doi:10.1016/0022-2364(85)90104-0
Pintacuda G, John M, Su XC, Otting G (2007) NMR structure determination of protein-ligand complexes by lanthanide labeling. Acc Chem Res 40:206–212. doi:10.1021/ar050087z
Ranganathan RS, Raju N, Fan H, Zhang X, Tweedle MF, Desreux JF, Jacques V (2002) Polymethylated DOTA ligands. 2. Synthesis of rigidified lanthanide chelates and studies on the effect of alkyl substitution on conformational mobility and relaxivity. Inorg Chem 41:6856–6866. doi:10.1021/ic025695e
Rodriguez-Castañeda F, Haberz P, Leonov A, Griesinger C (2006) Paramagnetic tagging of diamagnetic proteins for solution NMR. Magn Reson Chem 44:S10–S16. doi:10.1002/mrc.1811
Rudovský J et al (2005) Lanthanide(III) complexes of a mono(methylphosphonate) analogue of H4dota: the influence of protonation of the phosphonate moiety on the TSAP/SAP isomer ratio and the water exchange rate. Chemistry 11:2373–2384. doi:10.1002/chem.200400367
Schrödinger L (2010) The PyMOL molecular graphics system. Version 1.5. Schrödinger LLC, New York
Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM (2003) The Xplor-NIH NMR molecular structure determination package. J Magn Reson 160:65–73. doi:10.1016/S1090-7807(02)00014-9
Schwieters CD, Kuszewski JJ, Clore GM (2006) Using Xplor-NIH for NMR molecular structure determination. Prog Nucl Magn Res Spectrosc 48:47–62. doi:10.1016/j.pnmrs.2005.10.001
Vranken WF et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins Struct Funct Bioinform 59:687–696. doi:10.1002/prot.20449
Williamson MP (2013) Using chemical shift perturbation to characterise ligand binding. Prog Nucl Magn Reson Spectrosc 73:1–16. doi:10.1016/j.pnmrs.2013.02.001
Woods M, Sherry AD (2003) Synthesis and luminescence studies of aryl substituted tetraamide complexes of europium(III): a new approach to pH responsive luminescent europium probes. Inorg Chem 42:4401–4408. doi:10.1021/ic0300823
Woods M, Kovacs Z, Zhang S, Sherry AD (2003) Towards the rational design of magnetic resonance imaging contrast agents: isolation of the two coordination isomers of lanthanide DOTA-type complexes. Angew Chem Int Ed Engl 42:5889–5892. doi:10.1002/anie.200352234
Woods M, Botta M, Avedano S, Wang J, Sherry AD (2005) Towards the rational design of MRI contrast agents: a practical approach to the synthesis of gadolinium complexes that exhibit optimal water exchange. Dalton Trans 24:3829–3837. doi:10.1039/b510778d
Woods M et al (2011) Investigations into whole water, prototropic and amide proton exchange in lanthanide(III) DOTA-tetraamide chelates. Dalton Trans 40:6759–6764. doi:10.1039/c1dt10616c
Yang F, Wang X, Pan B-B, Su XC (2016) Single-armed phenylsulfonated pyridine derivative of DOTA is a rigid and stable paramagnetic tag in protein analysis. Chem Commun (Camb). doi:10.1039/C6CC06114A
Zhang S, Winter P, Wu K, Sherry AD (2001a) A novel europium(III)-based MRI contrast agent. J Am Chem Soc 123:1517–1518. doi:10.1021/ja005820q
Zhang S, Wu K, Biewer MC, Sherry AD (2001b) 1H and 17O NMR detection of a lanthanide-bound water molecule at ambient temperatures in pure water as solvent. Inorg Chem 40:4284–4290. doi:10.1021/ic0003877
Zhang S, Michaudet S, Burgess S, Sherry AD (2002) The amide protons of an ytterbium(III) dota tetraamide complex act as efficient antennae for transfer of magnetization to bulk water. Angew Chem Int Ed Engl 41:1919–1921. doi:10.1002/1521-3773(20020603)41:11<1919:AID-ANIE1919>3.0.CO;2-Q
Zhang S, Merritt M, Woessner DE, Lenkinski RE, Sherry AD (2003) PARACEST agents: modulating MRI contrast via water proton exchange. Acc Chem Res 36:783–790. doi:10.1021/ar020228m
Acknowledgments
The project was funded by the Intramural Research Programs of the National Heart, Lung, and Blood Institute and the Center for Information Technology of the NIH. We also thank Dr. Duck-Yeon Lee of the Biochemical Core Facility, National Heart, Lung, and Blood Institute for expertise and advice regarding mass spectrometry-related experiments. We appreciate the assistance of Dr. James Ferretti in acquiring the 17O NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strickland, M., Schwieters, C.D., Göbl, C. et al. Characterizing the magnetic susceptibility tensor of lanthanide-containing polymethylated-DOTA complexes. J Biomol NMR 66, 125–139 (2016). https://doi.org/10.1007/s10858-016-0061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-016-0061-x