Abstract
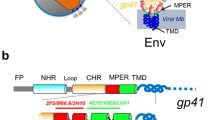
The envelope glycoprotein gp41 mediates the process of membrane fusion that enables entry of the HIV-1 virus into the host cell. Strong lipid affinity of the ectodomain suggests that its heptad repeat regions play an active role in destabilizing membranes by directly binding to the lipid bilayers and thereby lowering the free-energy barrier for membrane fusion. In such a model, immediately following the shedding of gp120, the N-heptad and C-heptad helices dissociate and melt into the host cell and viral membranes, respectively, pulling the destabilized membranes into juxtaposition, ready for fusion. Post-fusion, reaching the final 6-helix bundle (6HB) conformation then involves competition between intermolecular interactions needed for formation of the symmetric 6HB trimer and the membrane affinity of gp41’s ectodomain, including its membrane-proximal regions. Our solution NMR study of the structural and dynamic properties of three constructs containing the ectodomain of gp41 with and without its membrane-proximal regions suggests that these segments do not form inter-helical interactions until the very late steps of the fusion process. Interactions between the polar termini of the heptad regions, which are not associating with the lipid surface, therefore may constitute the main driving force initiating formation of the final post-fusion states. The absence of significant intermolecular ectodomain interactions in the presence of dodecyl phosphocholine highlights the importance of trimerization of gp41’s transmembrane helix to prevent complete dissociation of the trimer during the course of fusion.






Similar content being viewed by others
References
Banerjee K, Weliky DP (2014) Folded monomers and hexamers of the ectodomain of the HIV gp41 membrane fusion protein: potential roles in fusion and synergy between the fusion peptide, hairpin, and membrane-proximal external region. Biochemistry. doi:10.1021/bi501159w
Bartesaghi A, Merk A, Borgnia MJ, Milne JLS, Subramaniam S (2013) Prefusion structure of trimeric HIV-1 envelope glycoprotein determined by cryo-electron microscopy. Nat Struct Mol Biol 20:1352–1357
Blumenthal R, Durell S, Viard M (2012) HIV entry and envelope glycoprotein-mediated fusion. J Biol Chem 287:40841–40849
Buzon V, Natrajan G, Schibli D, Campelo F, Kozlov MM, Weissenhorn W (2010) Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog 6:7
Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM (1998) Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J 17:4572–4584
Carr CM, Kim PS (1994) Flu virus invasion: halfway there. Science 266:234–236
Cavanagh J, Fairbrother WJ, Palmer AG, Rance M, Skelton N (2007) Protein NMR spectroscopy: principles and practice. Elsevier Academic Press, Burlington
Chan DC, Fass D, Berger JM, Kim PS (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273
Chen J, Wharton SA, Weissenhorn W, Calder LJ, Hughson FM, Skehel JJ, Wiley DC (1995) A soluble domain of the membrane-anchoring chain of influenza virus hemagglutinin (HA(2)) folds in Escherichia coli into the low-pH-induced conformation. Proc Natl Acad Sci USA 92:12205–12209
Clore GM, Szabo A, Bax A, Kay LE, Driscoll PC, Gronenborn AM (1990) Deviations from the simple two-parameter model-free approach to the interpretation of nitrogen-15 nuclear magnetic relaxation of proteins. J Am Chem Soc 112:4989–4991
Dong H, Sharma M, Zhou H-X, Cross TA (2012) Glycines: role in alpha-helical membrane protein structures and a potential indicator of native conformation. Biochemistry 51:4779–4789
Durrer P, Galli C, Hoenke S, Corti C, Gluck R, Vorherr T, Brunner J (1996) H+-induced membrane insertion of influenza virus hemagglutinin involves the HA2 amino-terminal fusion peptide but not the coiled coil region. J Biol Chem 271:13417–13421
Epand RF, Macosko JC, Russell CJ, Shin YK, Epand RM (1999) The ectodomain of HA2 of influenza virus promotes rapid pH dependent membrane fusion. J Mol Biol 286:489–503
Furuta RA, Wild CT, Weng YK, Weiss CD (1998) Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol 5:276–279
Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R (2003) The HIV Env-mediated fusion reaction. Biochim Biophys Acta 1614:36–50
Gao G, Wieczorek L, Peachman KK, Polonis VR, Alving CR, Rao M, Rao VB (2013) Designing a soluble near full-length HIV-1 gp41 Trimer. J Biol Chem 288:234–246
Harrison SC (2008) Viral membrane fusion. Nat Struct Mol Biol 15:690–698
Henderson R (2013) Avoiding the pitfalls of single particle cryo-electron microscopy: einstein from noise. Proc Natl Acad Sci USA 110:18037–18041
Hildinger M, Dittmar MT, Schult-Dietrich P, Fehse B, Schnierle BS, Thaler S, Stiegler G, Welker R, von Laer D (2001) Membrane-anchored peptide inhibits human immunodeficiency virus entry. J Virol 75:3038–3042
Hollmann A, Matos PM, Augusto MT, Castanho MARB, Santos NC (2013) Conjugation of cholesterol to HIV-1 fusion inhibitor C34 increases peptide-membrane interactions potentiating its action. PLoS ONE 8:e60302
Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A (2005) Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry 44:16167–16180
Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA (2013) Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483
Kim JH, Hartley TL, Curran AR, Engelman DM (2009) Molecular dynamics studies of the transmembrane domain of gp41 from HIV-1. Biochim Biophys Acta 1788:1804–1812
Kliger Y, Peisajovich SG, Blumenthal R, Shai Y (2000) Membrane-induced conformational change during the activation of HIV-1 gp41. J Mol Biol 301:905–914
Kondo N, Miyauchi K, Meng F, Iwamoto A, Matsuda Z (2010) Conformational changes of the HIV-1 envelope protein during membrane fusion are inhibited by the replacement of its membrane-spanning domain. J Biol Chem 285:14681–14688
Korazim O, Sackett K, Shai Y (2006) Functional and structural characterization of HIV-1 gp41 ectodomain regions in phospholipid membranes suggests that the fusion-active conformation is extended. J Mol Biol 364:1103–1117
Lakomek NA, Ying JF, Bax A (2012) Measurement of 15 N relaxation rates in perdeuterated proteins by TROSY-based methods. J Biomol NMR 53:209–221
Lakomek N-A, Kaufman JD, Stahl SJ, Louis JM, Grishaev A, Wingfield PT, Bax A (2013) Internal dynamics of the homotrimeric HIV-1 viral coat protein gp41 on multiple time scales. Angew Chem Int Ed 52:3911–3915
Lakomek N-A, Kaufman JD, Stah SJ, Wingfield PT (2014) HIV-1 envelope protein gp41: an NMR study of dodecyl phosphocholine embedded gp41 reveals a dynamic prefusion intermediate conformation. Structure 22:1311–1321
Lev N, Fridmann-Sirkis Y, Blank L, Bitler A, Epand RF, Epand RM, Shai Y (2009) Conformational stability and membrane interaction of the full-length ectodomain of HIV-1 gp41: implication for mode of action. Biochemistry 48:3166–3175
Lipari G, Szabo A (1982) Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559
Lorieau JL, Louis JM, Bax A (2011) Whole-body rocking motion of a fusion peptide in lipid bilayers from size-dispersed 15 N NMR relaxation. J Am Chem Soc 133:14184–14187
Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB (2013) Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science 342:1484–1490
MacKenzie KR, Prestegard JH, Engelman DM (1997) A transmembrane helix dimer: structure and implications. Science 276:131–133
Mao Y, Castillo-Menendez LR, Sodroski JG (2013a) Reply to subramaniam, van Heel, and Henderson: validity of the cryo- electron microscopy structures of the HIV-1 envelope glycoprotein complex. Proc Natl Acad Sci USA 110:E4178–E4182
Mao Y, Wang L, Gu C, Herschhorn A, Desormeaux A, Finzi A, Xiang S-H, Sodroski JG (2013b) Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer. Proc Natl Acad Sci USA 110:12438–12443
Markosyan RM, Cohen FS, Melikyan GB (2003) HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol Biol Cell 14:926–938
Melikyan GB (2014) HIV entry: a game of hide-and-fuse? Curr Opin Virol 4:1–7
Melikyan GB, Egelhofer M, von Laer D (2006) Membrane-anchored inhibitory peptides capture human immunodeficiency virus type 1 gp41 conformations that engage the target membrane prior to fusion. J Virol 80:3249–3258
Merk A, Subramaniam S (2013) HIV-1 envelope glycoprotein structure. Curr Opin Struct Biol 23:268–276
Miyauchi, K, Curran, AR, Long, Y, Kondo, N, Iwamoto, A, Engelman, DM, Matsuda, Z (2010) The membrane-spanning domain of gp41 plays a critical role in intracellular trafficking of the HIV envelope protein. Retrovirology 7:95
Ratnayake PU, Sackett K, Nethercott MJ, Weliky DP (2015) pH-dependent vesicle fusion induced by the ectodomain of the human immunodeficiency virus membrane fusion protein gp41: two kinetically distinct processes and fully-membrane-associated gp41 with predominant β sheet fusion peptide conformation. Biochim Biophys Acta 1848:289–298
Reardon PN, Sage H, Dennison SM, Martin JW, Donald BR, Alam SM, Haynes BF, Spicer LD (2014) Structure of an HIV-1-neutralizing antibody target, the lipid-bound gp41 envelope membrane proximal region trimer. Proc Natl Acad Sci USA 111:1391–1396
Reuven EM, Dadon Y, Viard M, Manukovsky N, Blumenthal R, Shai Y (2012) HIV-1 gp41 transmembrane domain interacts with the fusion peptide: implication in lipid mixing and inhibition of virus-cell fusion. Biochemistry 51:2867–2878
Roche J, Louis JM, Grishaev A, Ying J, Bax A (2014) Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion. Proc Natl Acad Sci USA 111:3425–3430
Roux KH, Taylor KA (2007) AIDS virus envelope spike structure. Curr Opin Struct Biol 17:244–252
Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919
Sackett K, Shai Y (2002) The HIV-1 gp41 N-terminal heptad repeat plays an essential role in membrane fusion. Biochemistry 41:4678–4685
Sackett K, Nethercott MJ, Shai Y, Weliky DP (2009) Hairpin folding of HIV gp41 abrogates lipid mixing function at physiologic pH and inhibits lipid mixing by exposed gp41 constructs. Biochemistry 48:2714–2722
Sackett K, TerBush A, Weliky DP (2011) HIV gp41 six-helix bundle constructs induce rapid vesicle fusion at pH 3.5 and little fusion at pH 7.0: understanding pH dependence of protein aggregation, membrane binding, and electrostatics, and implications for HIV-host cell fusion. Eur Biophys J Biophys Lett 40:489–502
Sackett K, Nethercott MJ, Zheng Z, Weliky DP (2014) Solid-state NMR spectroscopy of the HIV gp41 membrane fusion protein supports intermolecular antiparallel 13 sheet fusion peptide structure in the final six-helix bundle state. J Mol Biol 426:1077–1094
Skehel JJ, Wiley DC (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69:531–569
Smith EC, Smith SE, Carter JR, Webb SR, Gibson KM, Hellman LM, Fried MG, Dutch RE (2013) Trimeric transmembrane domain interactions in paramyxovirus fusion proteins. Roles in protein folding, stability, and function. J Biol Chem 288:35726–35735
Subramaniam S (2013) Structure of trimeric HIV-1 envelope glycoproteins. Proc Natl Acad Sci USA 110:E4172–E4174
Tamm LK, Lee J, Liang B (2014) Capturing glimpses of an elusive HIV Gp41 prehairpin fusion intermediate. Structure 22:1225–1226
Tan KM, Liu JH, Wang JH, Shen S, Lu M (1997) Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA 94:12303–12308
Tatulian SA, Tamm LK (2000) Secondary structure, orientation, oligomerization, and lipid interactions of the transmembrane domain of influenza hemagglutinin. Biochemistry 39:496–507
van Heel M (2013) Finding trimeric HIV-1 envelope glycoproteins in random noise. Proc Natl Acad Sci USA 110:E4175–E4177
Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430
Yu YG, King DS, Shin YK (1994) Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science 266:274–276
Acknowledgments
We thank Drs. James Baber and Jinfa Ying for technical support and acknowledge support from the Advanced Mass Spectrometry Core of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This work was funded by the NIH Intramural Research Program of the NIDDK and by the Intramural AIDS-Targeted Antiviral Program of the Office of the Director, NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roche, J., Louis, J.M., Aniana, A. et al. Complete dissociation of the HIV-1 gp41 ectodomain and membrane proximal regions upon phospholipid binding. J Biomol NMR 61, 235–248 (2015). https://doi.org/10.1007/s10858-015-9900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-015-9900-4