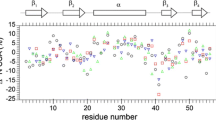
Abstract
The protein amide 1HN chemical shift temperature coefficient can be determined with high accuracy by recording spectra at different temperatures, but the physical mechanism responsible for this temperature dependence is not well understood. In this work, we find that this coefficient strongly correlates with the temperature coefficient of the through-hydrogen-bond coupling, 3hJNC′, based on NMR measurements of protein GB3. Parallel tempering molecular dynamics simulation suggests that the hydrogen bond distance variation at different temperatures/replicas is largely responsible for the 1HN chemical shift temperature dependence, from which an empirical equation is proposed to predict the hydrogen bond thermal expansion coefficient, revealing responses of individual hydrogen bonds to temperature changes. Different expansion patterns have been observed for various networks formed by β strands.






Similar content being viewed by others
References
Andersen NH et al (1997) Extracting information from the temperature gradients of polypeptide NH chemical shifts.1. The importance of conformational averaging. J Am Chem Soc 119:8547–8561
Barfield M (2002) Structural dependencies of interresidue scalar coupling (h3)J(NC), and donor H-1 chemical shifts in the hydrogen bonding regions of proteins. J Am Chem Soc 124:4158–4168
Baxter NJ, Williamson MP (1997) Temperature dependence of H-1 chemical shifts in proteins. J Biomol NMR 9:359–369
Berendsen HJC (1991) Transport-properties computed by linear response through weak-coupling to a bath. Nato Adv Sci I E App 205:139–155
Cavalli A et al (2007) Protein structure determination from NMR chemical shifts. Proc Natl Acad Sci USA 104:9615–9620
Cierpicki T, Otlewski J (2001) Amide proton temperature coefficients as hydrogen bond indicators in proteins. J Biomol NMR 21:249–261
Cierpicki T et al (2002) Hydrogen bonds in human ubiquitin reflected in temperature coefficients of amide protons. J Magn Reson 157:178–180
Cordier F, Grzesiek S (1999) Direct observation of hydrogen bonds in proteins by interresidue (3 h)J(NC′) scalar couplings. J Am Chem Soc 121:1601–1602
Cordier F, Grzesiek S (2002) Temperature-dependence properties as studied by of protein hydrogen bond high-resolution NMR. J Mol Biol 317:739–752
Cornilescu G, Hu JS, Bax A (1999a) Identification of the hydrogen bonding network in a protein by scalar couplings. J Am Chem Soc 121:2949–2950
Cornilescu G et al (1999b) Correlation between (3 h)J(NC′) and hydrogen bond length in proteins. J Am Chem Soc 121:6275–6279
Darden T, York D, Pedersen L (1993) Particle Mesh Ewald—an N.Log(N) method for Ewald Sums in large systems. J Chem Phys 98:10089–10092
Essmann U et al (1995) A smooth particle mesh ewald method. J Chem Phys 103:8577–8593
Grzesiek S et al (2004) Insights into biomolecular hydrogen bonds from hydrogen bond scalar couplings. Prog Nucl Magn Reson Spectrosc 45:275–300
Hansmann UHE (1997) Parallel tempering algorithm for conformational studies of biological molecules. Chem Phys Lett 281:140–150
Hess B et al (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18:1463–1472
Hess B et al (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447
Hornak V et al (2006) Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins Struct Funct Bioinform 65:712–725
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph Model 14:33–38
Kjaergaard M, Brander S, Poulsen FM (2011) Random coil chemical shift for intrinsically disordered proteins: effects of temperature and pH. J Biomol NMR 49:139–149
Kohlhoff KJ et al (2009) Fast and accurate predictions of protein nmr chemical shifts from interatomic distances. J Am Chem Soc 131:13894–13895
Miyamoto S, Kollman PA (1992) Settle: an analytical version of the shake and rattle algorithm for rigid water models. J Comput Chem 13:952–962
Neal S et al (2003) Rapid and accurate calculation of protein H-1, C-13 and N-15 chemical shifts. J Biomol NMR 26:215–240
Ohnishi M, Urry DW (1969) Temperature dependence of amide proton chemical shift: secondary structures of gramicidin s and valinomycin. Biochem Bioph Res Co 36:194–202
Parker LL, Houk AR, Jensen JH (2006) Cooperative hydrogen bonding effects are key determinants of backbone amide proton chemical shifts in proteins. J Am Chem Soc 128:9863–9872
Patriksson A, van der Spoel D (2008) A temperature predictor for parallel tempering simulations. Phys Chem Chem Phys 10:2073–2077
Sass H-J, Schmid FF-F, Grzesiek S (2007) Correlation of protein structure and dynamics to scalar couplings across hydrogen bonds. J Am Chem Soc 129:5898–5903
Shen Y, Bax A (2007) Protein backbone chemical shifts predicted from searching a database for torsion angle and sequence homology. J Biomol NMR 38:289–302
Shen Y, Bax A (2010) SPARTA plus: a modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. J Biomol NMR 48:13–22
Shen Y et al (2008) Consistent blind protein structure generation from NMR chemical shift data. Proc Natl Acad Sci USA 105:4685–4690
Shen Y et al (2009) TALOS plus: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223
Sitkoff D, Case DA (1997) Density functional calculations of proton chemical shifts in model peptides. J Am Chem Soc 119:12262–12273
Sugita Y, Okamoto Y (1999) Replica-exchange molecular dynamics method for protein folding. Chem Phys Lett 314:141–151
Tomlinson JH, Williamson MP (2012) Amide temperature coefficients in the protein G B1 domain. J Biomol NMR 52:57–64
Van der Spoel D et al (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718
Vila JA et al (2008) Quantum chemical C-13(alpha) chemical shift calculations for protein NMR structure determination, refinement, and validation. Proc Natl Acad Sci USA 105:14389–14394
Xu XP, Case DA (2001) Automated prediction of (15)N, (13)C(alpha), (13)C(beta) and (13)C′ chemical shifts in proteins using a density functional database. J Biomol NMR 21:321–333
Yao L, Ying J, Bax A (2009) Improved accuracy of N-15-H-1 scalar and residual dipolar couplings from gradient-enhanced IPAP-HSQC experiments on protonated proteins. J Biomol NMR 43:161–170
Acknowledgments
The authors would like to thank Dr. Dennis Torchia for the critical reading of the manuscript and Shanghai supercomputer center for the computer resources. This work was supported in part by 100 Talent Project of Chinese Academy of Sciences, National Nature Science Foundation of China (Grant no. 21173247) and the Foundation for Outstanding Young Scientist in Shandong Province (Grant no. JQ201104).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong, J., Jing, Q. & Yao, L. The protein amide 1HN chemical shift temperature coefficient reflects thermal expansion of the N–H···O=C hydrogen bond. J Biomol NMR 55, 71–78 (2013). https://doi.org/10.1007/s10858-012-9689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-012-9689-3