Abstract
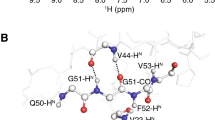
Using the case of the catalytic domain of MMP-12 in complex with the known inhibitor CGS27023A, a recently assembled 3D 15N-edited/14N,12C-filtered ROESY experiment is used to monitor and distinguish protein amide protons in fast exchange with bulk water from amide protons close to water molecules with longer residence times, the latter possibly reflecting water molecules of structural or functional importance. The 15N-edited/14N,12C-filtered ROESY spectra were compared to the original 15N-edited/14N,12C-filtered NOESY and the conventional amide-water exchange experiment, CLEANEX. Three protein backbone amide protons experiencing direct dipolar cross relaxation with water in the 15N-edited/14N,12C-filtered ROESY spectrum were assigned. In an ensemble of six crystal structures, two conserved water molecules within 3 Å of the three amide protons were identified. These two water molecules are buried into cavities in the protein surface and thus sufficiently slowed down by the protein topology to account for the observed dipolar interaction. Structural analysis of an ensemble of six crystal structures ruled out any exchange-relayed contributions for the amide-water interactions of interest.




Similar content being viewed by others
Abbreviations
- MMP:
-
Matrix metalloprotease
- NMR:
-
Nuclear magnetic resonance
- NOESY:
-
Nuclear overhauser effect spectroscopy
- ROESY:
-
Rotating frame overhauser effect spectroscopy
References
Badger J (1997) Modeling and refinement of water molecules and disordered solvent. Methods Enzymol 277B:344–352
Bertini I, Calderone V, Cosenza M, Fragai M, Lee Y-M, Luchinat C, Mangani S, Terni B, Turano P (2005) Conformational variability of matrix metalloproteinases: beyond a single 3D structure. Proc Natl Acad Sci USA 102:5334–5339
Bertini I, Calderone V, Fragai M, Giachetti A, Loconte M, Luchinat C, Maletta M, Nativi C, Yeo KJ (2007) Exploring the subtleties of drug-receptor interactions: the case of matrix metalloproteinases. J Am Chem Soc 129:2466–2475
Böckmann A, Guittet E (1996) Suppression of radiation damping during selective excitation of the water signal: the WANTED sequence. J Biomol NMR 8:87–92
Borsi V, Calderone V, Fragai M, Luchinat C, Sarti N (2010) Entropic contribution to the linking coefficient in fragment based drug design: a case study. J Med Chem 53:4285–4289
Clore GM, Bax A, Wingfield PT, Gronenborn AM (1990) Identification and localization of bound internal water in the solution structure of interleukin 1.beta. by heteronuclear three-dimensional proton rotating-frame Overhauser nitrogen-15-proton multiple quantum coherence NMR spectroscopy. Biochemistry 29:5671–5676
Dalvit C, Hommel U (1995) Sensitivity-improved detection of protein hydration and its extension to the assignment of fast-exchanging resonances. J Magn Reson B 109:334–338
Devel L, Garcia S, Czarny B, Beau F, Lajeunesse E, Vera L, Georgiadis D, Stura E, Dive V (2006) Insights from selective non-phosphinic inhibitors of MMP-12 tailored to fit with an S1 loop canonical conformation. J Biol Chem 285:35900–35909
Grzesiek S, Bax A (1993) Measurement of amide proton exchange rates and NOEs with water in 13C/15 N-enriched calcineurin B. J Biomol NMR 3:627–638
Halle B (2002) Flexibility and packing in proteins. Proc Natl Acad Sci USA 99:1274–1279
Halle B (2003) Cross-relaxation between macromolecular and solvent spins: the role of long-range dipole couplings. J Chem Phys 119:12372–12385
Halle B (2004) Protein hydration dynamics in solution: a critical survey. Philos Trans R Soc London, B 359:1323–1328
Holmes IP, Gaines S, Watson SP, Lorthioir O, Walker A, Baddeley SJ, Herbert S, Egan D, Convery MA, Singh OMP, Gross JW, Strelow JM, Smith RH, Amour AJ, Brown D, Martin SL (2009) The identification of [beta]-hydroxy carboxylic acids as selective MMP-12 inhibitors. Bioorg Med Chem Lett 19:5760–5763
Hwang T-L, Mori S, Shaka AJ, van Zijl PCM (1997) Application of phase-modulated CLEAN chemical EXchange spectroscopy (CLEANEX-PM) to detect water-protein proton exchange and intermolecular NOEs. J Am Chem Soc 119:6203–6204
Isaksson J, Nyström S, Derbyshire D, Agback T, Kovacs H, Bertini I, Giachetti A, Luchinat C (2009) Does a fast nuclear magnetic resonance spectroscopy- and X-ray crystallography hybrid approach provide reliable structural information of ligand-Protein complexes? A case study of metalloproteinases. J Med Chem 52:1712–1722
Islam SA, Weaver DL (1990) Molecular interactions in protein crystals: solvent accessible surface and stability. Proteins: Struct, Funct, Bioinf 8:1–5
MacPherson LJ, Bayburt EK, Capparelli MP, Carroll BJ, Goldstein R, Justice MR, Zhu L, Hu S, Melton RA, Fryer L, Goldberg RL, Doughty JR, Spirito S, Blancuzzi V, Wilson D, O’Byrne EM, Ganu V, Parker DT (1997) Discovery of CGS 27023A, a non-peptidic, potent, and orally active stromelysin inhibitor that blocks cartilage degradation in rabbits. J Med Chem 40:2525–2532
Modig K, Liepinsh E, Otting G, Halle B (2004) Dynamics of protein and peptide hydration. J Am Chem Soc 126:102–114
Morales R, Perrier S, Florent J-M, Beltra J, Dufour S, De Mendez I, Manceau P, Tertre A, Moreau F, Compere D, Dublanchet A-C, O’Gara M (2004) Crystal structures of novel non-peptidic, non-zinc chelating inhibitors Bound to MMP-12. J Mol Biol 341:1063–1076
Mori S, Berg JM, Zijl PCM (1996) Separation of intramolecular NOE and exchange peaks in water exchange spectroscopy using spin-echo filters. J Biomol NMR 7:77–82
Nar H, Werle K, Bauer MMT, Dollinger H, Jung B (2001) Crystal structure of human macrophage elastase (MMP-12) in complex with a hydroxamic acid inhibitor. J Mol Biol 312:743–751
Newby MI, Greenbaum NL (2002) Investigation of Overhauser effects between pseudouridine and water protons in RNA helices. Proc Natl Acad Sci USA 99:12697–12702
Nucci NV, Pometun MS, Wand AJ (2011) Site-resolved measurement of water-protein interactions by solution NMR. Nat Struct Mol Biol 18:245–249
Ohlendorf D (1994) Accuracy of refined protein structures. II. Comparison of four independently refined models of human interleukin 1[beta]. Acta Crystallogr Sect D: Biol Crystallogr 50:808–812
Otting G, Liepinsh E (1995) Selective excitation of intense solvent signals in the presence of radiation dampening. J Biomol NMR 5:420–426
Otting G, Liepinsh E, Farmer BT, Wüthrich K (1991a) Protein hydration studied with homonuclear 3D 1H NMR experiments. J Biomol NMR 1:209–215
Otting G, Liepinsh E, Wuthrich K (1991b) Protein hydration in aqueous solution. Science 254:974–980
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, vol 1, 2, 3. Cold Spring Harbor Laboratory Press, New York
Zwahlen C, Legault P, Vincent SJF, Greenblatt J, Konrat R, Kay LE (1997) Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: application to a bacteriophage N-peptide/boxB RNA complex. J Am Chem Soc 119:6711–6721
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovacs, H., Agback, T. & Isaksson, J. Probing water-protein contacts in a MMP-12/CGS27023A complex by nuclear magnetic resonance spectroscopy. J Biomol NMR 53, 85–92 (2012). https://doi.org/10.1007/s10858-012-9624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-012-9624-7