Abstract
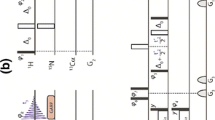
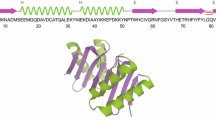
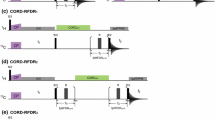
We describe a novel pulse sequence, MQ-HNCO-TROSY, for the measurement of scalar and residual dipolar couplings between amide proton and nitrogen in larger proteins. The experiment utilizes the whole 2TN polarization transfer delay for labeling of 15N chemical shift in a constant time manner, which efficiently doubles the attainable resolution in 15N dimension with respect to the conventional HNCO-TROSY experiment. In addition, the accordion principle is employed for measuring (J + D)NHs, and the multiplet components are selected with the generalized version of the TROSY scheme introduced by Nietlispach (J Biomol NMR 31:161–166, 2005). Therefore, cross peak overlap is diminished while the time period during which the 15N spin is susceptible to fast transverse relaxation associated with the anti-TROSY transition is minimized per attainable resolution unit. The proposed MQ-HNCO-TROSY scheme was employed for measuring RDCs in high molecular weight protein IgFLNa16-21 of 557 residues, resulting in 431 experimental RDCs. Correlations between experimental and back-calculated RDCs in individual domains gave relatively low Q-factors (0.19–0.39), indicative of sufficient accuracy that can be obtained with the proposed MQ-HNCO-TROSY experiment in high molecular weight proteins.







Similar content being viewed by others
References
Abrogast L, Majumdar A, Tolman JR (2010) HNCO-based measurement of one-bond amide 15N–1H couplings with optimized precision. J Biomol NMR 46:175–189
Andersson P, Annila A, Otting G (1998) An α/β-HSQC-α/β experiment for spin-state selective editing of IS cross peaks. J Magn Reson 133:364–367
Annila A, Permi P (2004) Weakly aligned biological macromolecules in dilute aqueous liquid crystals. Concepts Magn Reson 23A:22–37
Bax A, Kontaxis G, Tjandra N (2001) Dipolar couplings in macromolecular structure determination. Methods Enzymol 339:127–174
Blackledge M (2005) Recent progress in the study of biomolecular structure and dynamics in solution from residual dipolar couplings. Prog Nucl Magn Reson Spectr 46:23–61
Bodenhausen G, Ernst RR (1981) The accordion experiment, a simple approach to 3-dimensional NMR spectroscopy. J Magn Reson 45:367–373
Bouvignies G, Markwick PRL, Blackledge M (2007) Simultaneous definition of high resolution protein structure and backbone conformational dynamics using NMR residual dipolar couplings. ChemPhysChem 8:1901–1909
Düx P, Whitehead B, Boelens R, Kaptein R, Vuister GW (1999) Measurement of 15N–1H coupling constants in uniformly 15N-labeled proteins: application to the photoactive yellow protein. J Biomol NMR 10:301–306
Fischer MW, Losonczi JA, Weaver JL, Prestegard JH (1999) Domain orientation and dynamics in multidomain protein from residual dipolar couplings. Biochemistry 38:9013–9022
Fredriksson K, Louhivuori M, Permi P, Annila A (2004) On the interpretation of residual dipolar couplings as reporters of molecular dynamics. J Am Chem Soc 126:12646–12650
Hansen MR, Mueller L, Pardi A (1998) Tunable alignment of macromolecules by filamentous phage yields dipolar coupling interactions. Nat Struct Biol 5:1065–1074
Heikkinen S, Aitio H, Permi P, Folmer R, Lappalainen K, Kilpeläinen I (1999) J-multiplied HSQC (MJ-HSQC): a new method for measuring 3J(HNHα) couplings in 15N-labeled proteins. J Magn Reson 137:243–246
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J (2009) Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin. J Biol Chem 284:25450–25458
Hu K, Doucleff M, Clore GM (2009) Using multiple quantum-coherence to increase the 15N resolution in a three-dimensional TROSY-HNCO experiment for accurate PRE and RDC measurements. J Magn Reson 200:173–177
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient-enhanced heteronuclear single-quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Kontaxis G, Clore GM, Bax A (2000) Evaluation of cross-correlation effects and measurement of one-bond couplings in proteins with short transverse relaxation times. J Magn Reson 143:184–196
Lad Y, Kiema T, Jiang P, Pentikäinen O, Coles CH, Campbell ID, Calderwood DA, Ylänne J (2007) Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J 26:3993–4004
Lakomek NA, Carlomagno T, Becker S, Griesinger C, Meiler J (2006) A thorough dynamic interpretation of residual dipolar couplings in ubiquitin. J Biomol NMR 34:101–115
Lerche MH, Meissner A, Poulsen FM, Sørensen OW (1999) Pulse sequences for measurement of one-bond (15)N-(1)H coupling constants in the protein backbone. J Magn Reson 140:259–263
Losonczi JA, Andrec M, Fischer MWF, Prestegard JH (1999) Order matrix analysis of residual dipolar couplings using singular-value decomposition. J Magn Reson 138:334–342
Madsen JC, Sørensen OW, Sørensen P, Poulsen FM (1993) Improved pulse sequences for measuring coupling constants in 13C, 15N-labeled proteins. J Biomol NMR 3:239–244
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR-spectra without phase cycling—application to the study of hydrogen-exchange in proteins. J Magn Reson 85:393–399
McCoy MA, Mueller L (1992) Selective shaped pulse decoupling in NMR: homonuclear [13C]carbonyl decoupling. J Am Chem Soc 114:2108–2112
Meissner A, Duus JO, Sørensen OW (1997) Integration of spin-state-selective excitation into 2D NMR correlation experiments with the heteronuclear ZQ/DQ π rotations for 1JXH-resolved E.COSY-type measurement of heteronuclear coupling constants in proteins. J Biomol NMR 10:89–94
Nietlispach D (2005) Suppression of anti-TROSY lines in a sensitivity-enhanced gradient selection TROSY scheme. J Biomol NMR 31:161–166
Ottiger M, Delaglio F, Bax A (1998) Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson 131:373–378
Pääkkönen K, Sorsa T, Drakenberg T, Pollesello P, Tilgmann C, Permi P, Heikkinen S, Kilpeläinen I, Annila A (2000) Conformations of the regulatory domain of cardiac troponin C examined by residual dipolar couplings. Eur J Biochem 267:6665–6672
Permi P (2002) A spin-state-selective experiment for measuring heteronuclear one-bond and homonuclear two-bond couplings from an HSQC-type spectrum. J Biomol NMR 22:27–35
Permi P (2003) Measurement of residual dipolar couplings from 1Hα to 13Cα and 15N using a simple HNCA-based experiment. J Biomol NMR 27:341–349
Permi P, Rosevear PR, Annila A (2000a) A set of HNCO-based experiments for measurement of residual dipolar couplings in 15N, 13C, (2H) labeled proteins. J Biomol NMR 17:43–54
Permi P, Kilpeläinen I, Annila A (2000b) Determination of backbone angle ψ in proteins using a TROSY-based α/β-HN(CO)CA-J experiment. J Magn Reson 146:255–259
Pervushin K, Billeter M, Siegal G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole–dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures very large biological macromolecules in solution. Proc Natl Acad Sci U S A 94:12366–12371
Prestegard JH, Al-Hashimi HM, Tolman JR (2000) NMR structures of biomolecules using field oriented media and residual dipolar couplings. Quart Rev Biophys 33:371–424
Puttonen E, Tossavainen H, Permi P (2006) Simultaneous determination of one- and two-bond scalar and residual dipolar couplings between 13C′, 13Cα, and 15N spins in proteins. Magn Reson Chem 44:168–176
Salzmann M, Pervushin K, Wider G, Senn H, Wüthrich K (1998) TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc Natl Acad Sci U S A 95:13585–13590
Tjandra N, Bax A (1997) Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278:1111–1114
Tolman JR, Ruan K (2006) NMR residual dipolar couplings as probes of biomolecular dynamics. Chem Rev 106:1720–1736
Tugarinov V, Kay LE (2003) Quantitative NMR studies of high molecular weight proteins: application to domain orientation and ligand binding in the 723 residue enzyme malate synthase G. J Mol Biol 327:1121–1133
Wang AC, Bax A (1995) Reparametrization of the Karplus relation for 3J(Hα-N) and 3J(HN-C′) in peptides from uniformly 13C/15N-enriched human ubiquitin. J Am Chem Soc 117:1810–1813
Weigelt J (1998) Single scan, sensitivity- and gradient-enhanced TROSY for multidimensional NMR experiments. J Am Chem Soc 120:10778–10779
Yang DW, Kay LE (1999) Improved 1HN-detected triple resonance TROSY-based experiments. J Biomol NMR 13:3–10
Yang DW, Venters RA, Mueller GA, Choy WY, Kay LE (1999) TROSY-based HNCO pulse sequences for the measurement of 1HN-15N, 15N–13CO, 1HN-13CO, 13CO-13Ca and 1HN-13Ca dipolar couplings in 15N, 13C, 2H-labeled proteins. J Biomol NMR 14:333–343
Zweckstetter M, Bax A (2000) Prediction of sterically induced alignment in a dilute liquid crystalline phase: aid to protein structure determination by NMR. J Am Chem Soc 122:3791–3792
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mäntylahti, S., Koskela, O., Jiang, P. et al. MQ-HNCO-TROSY for the measurement of scalar and residual dipolar couplings in larger proteins: application to a 557-residue IgFLNa16-21. J Biomol NMR 47, 183–194 (2010). https://doi.org/10.1007/s10858-010-9422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-010-9422-z