Abstract
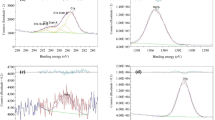
In an attempt to develop biodegradable, mechanically strong, biocompatible, and conductive nerve guidance conduits, pure magnesium (Mg) was used as the biodegradable substrate material to provide strength while the conductive polymer, poly(3,4-ethylenedioxythiophene) (PEDOT) was used as a conductive coating material to control Mg degradation and improve cytocompatibility of Mg substrates. This study explored a series of electrochemical deposition conditions to produce a uniform, consistent PEDOT coating on large three-dimensional Mg samples. A concentration of 1 M 3,4-ethylenedioxythiophene in ionic liquid was sufficient for coating Mg samples with a size of 5 × 5 × 0.25 mm. Both cyclic voltammetry (CV) and chronoamperometry coating methods produced adequate coverage and uniform PEDOT coating. Low-cost stainless steel and copper electrodes can be used to deposit PEDOT coatings as effectively as platinum and silver/silver chloride electrodes. Five cycles of CV with the potential ranging from −0.5 to 2.0 V for 200 s per cycle were used to produce consistent coatings for further evaluation. Scanning electron micrographs showed the micro-porous structure of PEDOT coatings. Energy dispersive X-ray spectroscopy showed the peaks of sulfur, carbon, and oxygen, indicating sufficient PEDOT coating. Adhesion strength of the coating was measured using the tape test following the ASTM-D 3359 standard. The adhesion strength of PEDOT coating was within the classifications of 3B to 4B. Tafel tests of the PEDOT coated Mg showed a corrosion current (ICORR) of 6.14 × 10−5 A as compared with ICORR of 9.08 × 10−4 A for non-coated Mg. The calculated corrosion rate for the PEDOT coated Mg was 2.64 mm/year, much slower than 38.98 mm/year for the non-coated Mg.








Similar content being viewed by others
References
Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–72.
Johnson I, Perchy D, Liu H. In vitro evaluation of the surface effects on magnesium-yttrium alloy degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2011;. doi:10.1002/jbm.a.33290.
Kainer KU, ed. Magnesium: proceedings of the 7th international conference on magnesium alloys and their applications; 2007. p 1126.
Muir KW. New experimental and clinical data on the efficacy of pharmacological magnesium infusions in cerebral infarcts. Magnes Res. 1998;11(1):43–56.
Clarkson AN. Anesthetic-mediated protection/preconditioning during cerebral ischemia. Life Sci. 2007;80(13):1157–75.
Gupta VK. Intravenous magnesium for neuroprotection in acute stroke: clinical hope versus basic neuropharmacology. Stroke. 2004;35(12):2758.
Saver JL, Kidwell C, Eckstein M, Starkman S, Investigators F-MPT. Prehospital neuroprotective therapy for acute stroke—Results of the field administration of stroke therapy-magnesium (FAST-MAG) pilot trial. Stroke. 2004;35(5):E106–8.
Campbell K, Meloni BP, Zhu HD, Knuckey NW. Magnesium treatment and spontaneous mild hypothermia after transient focal cerebral ischemia in the rat. Brain Res Bull. 2008;77(5):320–2.
Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6(5):1680–92.
Liu H. The effects of surface and biomolecules on magnesium degradation and mesenchymal stem cell adhesion. J Biomed Mater Res A. 2011;99(2):249–60.
Guan RG, Johnson I, Cui T, Zhao T, Zhao ZY, Li X, Liu H. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J Biomed Mater Res A. 2012;100(4):999–1015.
Luo XL, Cui XYT. Electrochemical deposition of conducting polymer coatings on magnesium surfaces in ionic liquid. Acta Biomater. 2011;7(1):441–6.
Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim DH, Martin DC. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials. 2007;28(8):1539–52.
Luo SC, Ali EM, Tansil NC, Yu HH, Gao S, Kantchev EAB, Ying JY. Poly(3,4-ethylenedioxythiophene) (PEDOT) nanobiointerfaces: thin, ultrasmooth, and functionalized PEDOT films with in vitro and in vivo biocompatibility. Langmuir. 2008;24(15):8071–7.
Cui XY, Martin DC. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sens Actuators B Chem. 2003;89(1–2):92–102.
Yang JY, Kim DH, Hendricks JL, Leach M, Northey R, Martin DC. Ordered surfactant-templated poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomater. 2005;1(1):125–36.
Bobacka J, Lewenstam A, Ivaska A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J Electroanal Chem. 2000;489(1–2):17–27.
Boretius T, Schuettler M, Stieglitz T. On the stability of poly-ethylenedioxythiopene as coating material for active neural implants. Artif Organs. 2011;35(3):245–8.
Urbanchek MG, Shim BS, Baghmanli Z, Wei B, Schroeder K, Langhals NB, Miriani RM, Egeland BM, Kipke, Martin DC, et al. Conduction properties of decellularized nerve biomaterials. IFMBE Proc. 2010;32:430–3.
Melanie Urbanchek MRA, Engeland B, Miriani RM, Schroeder K, Daneshvar E, Ewig K, Kuzon WM, Kipke DR, Cederna PS. Nerve Regeneration through PEDOT, an electrically conducting polymer nerve graft. Plast Surg; 2009. p. 124 (4S): 67.
Yang JY, Martin DC. Microporous conducting polymers on neural microelectrode arrays II Physical characterization. Sens Actuators A Phys. 2004;113(2):204–11.
Zhong YH, Bellamkonda RV. Biomaterials for the central nervous system. J R Soc Interface. 2008;5(26):957–75.
Tamburri E, Sarti S, Orlanducci S, Terranova ML, Rossi M. Study of PEDOT conductive polymer films by admittance measurements. Mater Chem Phys. 2011;125(3):397–404.
Chandrasekhar P. Conducting polymers fundamentals and applications. New York: Springer; 1999.
Macfarlane DR, Forsyth M, Howlett PC, Pringle JM, Sun J, Annat G, Neil W, Izgorodina EI. Ionic liquids in electrochemical devices and processes: managing interfacial electrochemistry. Acc Chem Res. 2007;40(11):1165–73.
Peter J, Blau KGB. Development and use of ASTM standards for wear testing. Wear. 1999;225–229(Part 2):1159–70.
Muto MIT, Kobayashi K, Miyasaka T. Conductive polymer-based mesoscopic counterelectrodes for plastic dye-sensitized solar cells. Chem Lett. 2007;36(6):804–5.
Kozak A. Effect of deposition characteristics on electrochemically prepared PEDOT films. NNIN REU Research Accomplishments 2010:20–21.
Acknowledgments
This material is based upon study supported by the National Science Foundation under Grant 1125801. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors would also like to thank the University of California for financial support. We would like to thank the Central Facility for Advanced Microscopy and Microanalysis (CFAMM) at the University of California, Riverside, thank Drs. X. Luo, X.T. Cui, and V. Vullev for their advice on electrochemistry, and thank Mitch Boretz for proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meriam A. Sebaa and Shan Dhillon contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sebaa, M.A., Dhillon, S. & Liu, H. Electrochemical deposition and evaluation of electrically conductive polymer coating on biodegradable magnesium implants for neural applications. J Mater Sci: Mater Med 24, 307–316 (2013). https://doi.org/10.1007/s10856-012-4796-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4796-y