Abstract
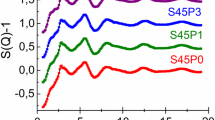
Melt quenched silicate glasses containing calcium, phosphorous and alkali metals have the ability to promote bone regeneration and to fuse to living bone. These glasses, including 45S5 Bioglass® [(CaO)26.9(Na2O)24.4(SiO2)46.1(P2O5)2.6], are routinely used as clinical implants. Consequently there have been numerous studies on the structure of these glasses using conventional diffraction techniques. These studies have provided important information on the atomic structure of Bioglass® but are of course intrinsically limited in the sense that they probe the bulk material and cannot be as sensitive to thin layers of near-surface dissolution/growth. The present study therefore uses surface sensitive shallow angle X-ray diffraction to study the formation of amorphous calcium phosphate and hydroxyapatite on Bioglass® samples, pre-reacted in simulated body fluid (SBF). Unreacted Bioglass® is dominated by a broad amorphous feature around 2.2 Å−1 which is characteristic of sodium calcium silicate glass. After reacting Bioglass® in SBF a second broad amorphous feature evolves ~1.6 Å−1 which is attributed to amorphous calcium phosphate. This feature is evident for samples after only 4 h reacting in SBF and by 8 h the amorphous feature becomes comparable in magnitude to the background signal of the bulk Bioglass®. Bragg peaks characteristic of hydroxyapatite form after 1–3 days of reacting in SBF.





Similar content being viewed by others
References
L.L. Hench, R.J. Splinter, W.C. Allen, T.K. Greenlee, J. Biomed. Mater. Res. 5(6), 117 (1971). doi:10.1002/jbm.820050611
A.E. Clark, L.L. Hench, H.A. Paschall, J. Biomed. Mater. Res. 10, 161 (1976). doi:10.1002/jbm.820100202
L.L. Hench, J. Wilson, D.C. Greenspan, J. Aust. Ceram. Soc. 40, 1 (2004)
L.L. Hench, J. Mater. Sci. Mater. Med. 17, 967 (2006). doi:10.1007/s10856-006-0432-z
See www.novabone.com and www.novamin.com for commercial details
S.V. Dorozhkin, M. Epple, Angew. Chem. Int. Ed. 41, 3130 (2002). doi:10.1002/1521-3773(20020902)41:17<3130:AID-ANIE3130>3.0.CO;2-1
R.J. Newport, L.J. Skipper, D. Carta, D.M. Pickup, F.E. Sowrey, M.E. Smith, P. Saravanapavan, L.L. Hench, J. Mater. Sci. Mater. Med. 17, 1003 (2006). doi:10.1007/s10856-006-0436-8
L.J. Skipper, F.E. Sowrey, R. Rashid, R.J. Newport, Z. Lin, M.E. Smith, Phys. Chem. Glasses 46(4), 372 (2005)
V. FitzGerald, D.M. Pickup, D. Greenspan, G. Sarkar, J.J. Fitzgerald, K.M. Wetherall, R.M. Moss, J.R. Jones, R.J. Newport, Adv. Funct. Mater. 17, 3746 (2007). doi:10.1002/adfm.200700433
V. FitzGerald, D.M. Pickup, D. Carta, D. Greenspan, R.J. Newport, Phys. Chem. Glasses 48, 340 (2007)
V. FitzGerald, D.M. Pickup, D. Greenspan, K.M. Weatherall, R.M. Moss, J.R. Jones, R.J. Newport, Phys. Chem. Glasses (2008) (in press)
J.S. Rigden, R.J. Newport, G.J. Bushnell-Wye, J. Mater. Res. 12, 264 (1997). doi:10.1557/JMR.1997.0034
T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi, T. Yamamuro, J. Biomed. Mater. Res. 24(6), 721 (1990). doi:10.1002/jbm.820240607
International standard: ISO/FDIS 23317:2007
I. Rehman, J.C. Knowles, W. Bonfield, J. Biomed. Mater. Res. 41(1), 162 (1998). doi:10.1002/(SICI)1097-4636(199807)41:1<162:AID-JBM19>3.0.CO;2-P
V. FitzGerald, R.A. Martin, J.R. Jones, D. Qiu, K.M. Wetherall, R.M. Moss, R.J, Newport, J. Biomed. Mater. Res. A (in press). doi:10.1002/jbm.a.32206
H. Ohsato, I. Maki, Y. Takeuchi, Acta Crystallogr. C 41, 1575 (1985). doi:10.1107/S0108270185008617
L. Stork, P. Mueller, R. Dronskowski, J.R. Ortlepp, Z. Kristallogr. 220, 201 (2005). doi:10.1524/zkri.220.2.201.59118
M.M. Pereira, A.E. Clark, L.L. Hench, J. Am. Ceram. Soc. 78(9), 2463 (1995). doi:10.1111/j.1151-2916.1995.tb08686.x
Acknowledgements
The authors thank the EPSRC for financial support (Grant no. EP/E050611/1) SRS for beam time allocation, Dr. Tony Bell for assistance on station 9.1 and acknowledge use of the Chemical Database Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, R.A., Twyman, H., Qiu, D. et al. A study of the formation of amorphous calcium phosphate and hydroxyapatite on melt quenched Bioglass® using surface sensitive shallow angle X-ray diffraction. J Mater Sci: Mater Med 20, 883–888 (2009). https://doi.org/10.1007/s10856-008-3661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3661-5