Abstract
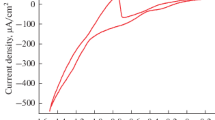
Hierarchical zinc oxide (ZnO) nanostructures were successfully grown by oxidation of etched Zn foil in water at 90 °C. The morphology of the ZnO nanostructures evolved from nanorods to hexagonal nanotubes then to flower-like structures with continued oxidation. Etching of the foil possibly promotes the growth of these ZnO nanostructures by creating inhomogeneities on the surface of the foil, which act as nucleation sites. In situ mixed potential measurement in combination with thermodynamic calculation was performed to elucidate the effect of temperature on the formation of the ZnO hierarchical nanostructures. The mixed potential increased with higher oxidation temperatures, suggesting enhanced oxidation.






Similar content being viewed by others
References
Ozgur U, Alivov YaI, Liu C, Teke A, Reshchikov MA, Dogan S, Avrutin V, Cho S-J, Morkoc H (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:041301. doi:10.1063/1.1992666
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys 16:R829–R858. doi:10.1088/0953-8984/16/25/R01
Hahn Y-B (2011) Zinc oxide nanostructures and their applications, Korean. J Chem Eng 28:1797–1813. doi:10.1007/s11814-011-0213-3
Nguyen XS, Tay CB, Fitzgerald EA, Chua SJ (2012) ZnO coaxial nanorod homojunction UV light-emitting diodes prepared by aqueous solution method. Small 8:1204–1208. doi:10.1002/smll.201102369
Govender K, Boyle DS, O’Brien P, Binks D, West D, Coleman D (2002) Room-temperature lasing observed from ZnO nanocolumns grown by aqueous solution deposition. Adv Mater 14:1221–1224. doi:10.1002/15214095(20020903)14:17<1221:AID-ADMA1221>3.0.CO;2-1
Fan ZY, Lu JG (2005) Electrical property of ZnO nanowires characterized by a scanning probe. Appl Phys Lett 86:032111–032113. doi:10.1063/1.1851621
Martinson ABF, Elam JW, Hupp JT, Pellin MJ (2007) ZnO nanotube based dye sensitized solar cells. Nano Lett 7:2183–2187. doi:10.1021/nl070160+
Zhou J, Gu Y, Hu Y, Mai W, Yeh P-H, Bao G, Sood AK, Polla DK, Wang ZL (2009) Gigantic enhancement in response and reset time of ZnO UV nanosensor by utilizing Schottky contact and surface functionalization. Appl Phys Lett 94:191103. doi:10.1063/1.3133358
Wang ZL (2007) Nanopiezotronics. Adv Mater 19:889–892. doi:10.1002/adma.200602918
Yang R, Qin Y, Dai L, Wang ZL (2009) Power generation with laterally packaged piezoelectric fine wires. Nat Nanotech 4:34–39. doi:10.1038/nnano.2008.314
Yin JZ, Gao F, Wei CZ, Lu QY (2014) Water amount dependence on morphologies and properties of ZnO nanostructures in double-solvent system. Sci Rep 4:3736. doi:10.1038/srep03736
Park JY, Park YK, Kim SS (2011) Formation of networked ZnO nanowires by vapor phase growth and their sensing properties with respect to CO. Mater Lett 65:2755–2757. doi:10.1016/j.matlet.2011.05.098
Qu X, Lu S, Wang J, Li Z, Xue H (2012) Preparation and optical property of porous ZnO nanobelts. Mater Sci Semicond Process 15:244–250. doi:10.1016/j.mssp.2011.08.007
Huang Y, Ke Y, Cui Q, Wang C, Zhang N, Zhu Z (2008) Low-temperature and two-step evaporation growth of ZnO nanotetrapods and their field emission properties. Mater Lett 62:1342–1344. doi:10.1016/j.matlet.2007.08.045
Liu WC, Cai W (2008) Synthesis and characterization of ZnO nanorings with ZnO nanowires array aligned at the inner surface without catalyst. J Cryst Growth 310:843–846. doi:10.1016/j.jcrysgro.2007.11.162
Liu Y, Gao W (2015) Growth process, crystal size and alignment of ZnO nanorods synthesized under neutral and acid conditions. J Alloy Compd 629:84–91. doi:10.1016/j.jallcom.2014.12.139
Huang Y, Zhang Y, He J, Dai Y, Gu Y, Ji Z, Zhou C (2006) Fabrication and characterization of ZnO comb-like nanostructures. Ceram Int 32:561–566. doi:10.1016/j.ceramint.2005.04.011
Roza L, Rahman MYA, Umar AA, Salleh MM (2015) Direct growth of oriented ZnO nanotubes by self-selective etching at lower temperature for photo-electrochemical (PEC) solar cell application. J Alloy Compd 618:153–158. doi:10.1016/j.jallcom.2014.08.113
Huang J, Ren H, Sun P, Gu C, Sun Y, Liu Y (2013) Facile synthesis of porous ZnO nanowires consisting of ordered nanocrystallites and their enhanced gas-sensing property. Sens Actuators B 188:249–256. doi:10.1016/j.snb.2013.06.103
Gao YF, Nagai M, Masuda Y, Sato F, Koumoto K (2006) Electrochemical deposition of ZnO film and its photoluminescence properties. J Cryst Growth 286:445–450. doi:10.1016/j.jcrysgro.2005.10.072
Zhu S, Shan L, Tian X, Zhang X, Sun D, Liu X, Wang L, Zhou Z (2014) Hydrothermal synthesis of oriented ZnO nanorod–nanosheets hierarchical architecture on zinc foil as flexible photoanodes for dye-sensitized solar cells. Ceram Int 40:11663–11670. doi:10.1016/j.ceramint.2014.03.173
Gao R, Cui Y, Liu X, Wang L, Cao G (2014) A ZnO nanorod/nanoparticle hierarchical structure synthesized through a facile in situ method for dye-sensitized solar cells. J Mater Chem A 2:4765–4770. doi:10.1039/C3TA15276F
Tan WK, Li LC, Razal KA, Kawamura G, Muto H, Matsuda A, Lockman Z (2014) Formation of two-dimensional ZnO nanosheets by rapid thermal oxidation in oxygenated environment. J Nanosci Nanotechnol 4:2960–2967. doi:10.1166/jnn.2014.8559
Sheini FJ, Joag DS, More MA (2010) Electrochemical synthesis of Sn doped ZnO nanowires on zinc foil and their field emission studies. Thin Solid Films 519:184–189. doi:10.1016/j.tsf.2010.07.106
Chang P-C, Fan Z, Wang D, Tseng W-Y, Chiou W-A, Hong J, Lu JG (2004) ZnO nanowires synthesized by vapor trapping CVD method. Chem Mater 16:5133–5137. doi:10.1021/cm049182c
Hsueh T-J, Hsu C-L, Chang S-J, Lin Y-R, Lin T-S, Chen I-C (2007) Growth and characterization of sparsely dispersed ZnO nanowire. J Electrochem Soc 54:H153–H156. doi:10.1149/1.2424415
Gomez JL, Tigli O (2013) Zinc oxide nanostructures: from growth to application”. J Mater Sci 48:612–624. doi:10.1007/s10853-012-6938-5
Matsuda A, Kotani Y, Kogure T, Tatsumisago M, Minami T (2000) Transparent Anatase Nanocomposite Films by the Sol-Gel Process at Low Temperatures. J Am Ceram Soc 83:229–231. doi:10.1111/j.1151-2916.2000.tb01178.x
Tan WK, Kawamura G, Muto H, Razak KA, Lockman Z, Matsuda A (2015) Blue-emitting photoluminescence of rod-like and needle-like ZnO nanostructures formed by hot-water treatment of sol–gel derived coatings. J Lumin 158:44–49. doi:10.1016/j.jlumin.2014.09.028
Prastomo N, Muto H, Sakai M, Matsuda A (2010) Formation and stabilization of tetragonal phase in sol–gel derived ZrO2 treated with base hot water. Mater Sci Eng B 173:99–104. doi:10.1016/j.mseb.2009.12.011
Tan WK, Razak KA, Lockman Z, Kawamura G, Muto H, Matsuda A (2013) Formation of highly crystallized ZnO nanostructures by hot-water treatment of etched Zn foils. Mater Lett 91:111–114. doi:10.1016/j.matlet.2012.08.103
Xi Y, Song J, Xu S, Yang R, Gao Z, Hu C, Wang ZL (2009) Growth of ZnO nanotube array and nanotube based piezoelectric nanogenerators. J Mater Chem 19:9260–9264. doi:10.1039/B917525C
Vayssieres L, Keis K, Hagfeldt A, Lindquist S-E (2001) Three-dimensional array of highly oriented crystalline ZnO microtubes. Chem Mater 13:4395–4398. doi:10.1021/cm011160s
Acknowledgements
This study was supported by the Department of Science and Technology under the Engineering Research and Development for Technology Scholarship Fund and the College of Engineering, University of the Philippines under the Faculty Research Incentive Award. Partial fund from Long Term Research Grant, Ministry of Education Malaysia, Project 2, 304/PBAHAN/6050235, Universiti Sains Malaysia is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Balela, M.D.L., Pelicano, C.M.O. & Lockman, Z. In situ mixed potential study of the growth of zinc oxide hierarchical nanostructures by wet oxidation of zinc foil. J Mater Sci 52, 2319–2328 (2017). https://doi.org/10.1007/s10853-016-0524-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0524-1