Abstract
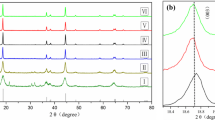
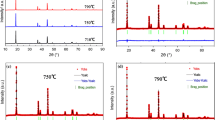
Layered LiNi0.8Co0.15Al0.05O2 cathode materials have been synthesized by co-precipitation methods. The effect of pre-thermal treatment was investigated by thermogravimetric differential thermal analysis. Although X-ray diffraction has confirmed that all diffraction peaks in XRD patterns for samples treated at 500 ~ 750 °C can be a well-indexed hexagonal structure, the status of nickel ions varied. Samples pre-treated at different temperatures show different colors and had various contents of Ni3+ measured by XPS. Powders that heated again at 800 °C under the condition of dried oxygen for 12 h after pre-thermal treatment show different electrochemical performances, which pre-thermal treated at 600 °C had a highest reversible specific capacity about 180 mAh·g−1 and capacity retention of 91.7 % after 50 cycles when cycled at a current density of 0.1 C between 2.5–4.3 V at room temperature. The relationship between the status of nickel ions and electrochemical performance was discussed. On the other hand, the capacity retention rates are 91.7, 96.6, and 98.0 % after 50 cycles at 0.1 C and at 100 %DOD, 80 DOD, and 50 %DOD.










Similar content being viewed by others
References
Choi YM, Pyun SI, Moon SI, Hyung YE (1998) A study of the electrochemical lithium intercalation behavior of porous LiNiO2 electrodes prepared by solid-state reaction and sol–gel methods. J Power Sources 72:83–90
Morales J, Vicente CP, Tirado JL (1990) Cation distribution and chemical deintercalation of Li1-xNi1+xO2. Mater Res Bull 25:623–630
Rougier A, Gravereau P, Delmas C (1996) Optimization of the composition of the Li1-zNi1+zO2 electrode materials: structural, magnetic, and electrochemical studies. J Electrochemical Soc 143:1168–1175
Dahn JR, Sacken UV, Michal CA (1990) Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni(OH)2 structure. Solid State Ionics 44:87–97
Kalaiselvi N, Raajaraajan AV, Sivagaminathan B, Renganathan NG, Muniyandi N, Ragavan M (2003) Synthesis of optimized LiNiO2 for Lithium ion batteries. Ionics 9:382–387
Lee KK, Yoon WS, Kim KB, Lee KY, Hong ST (2001) Thermal behavior and decomposition mechanism of electrochemically delithiated Li1-xNiO2. J Power Sources 97–98:321–325
Arai H, Okada S, Sakurai Y, Yamaki JI (1998) Thermal behavior of Li1-yNiO2 and the decomposition mechanism. Solid State Ionics 109:295–302
Stoyanova R, Zhecheva E, Kuzmanova E, Alcantara R, Lavela P, Tirado JL (2000) Aluminium coordination in LiNi1-yAlyO2 solid solutions. Solid State Ionics 128:1–10
Zhong QM, Sacken UV (1995) Crystal structures and electrochemical properties of LiAlyNil-yO2 solid solution. J Power Sources 54:221–223
Wang GX, Zhong S, Bradhurst DH, Dou SX, Liu HK (1999) LiAlδNi1-δO2 solid solutions as cathodic materials for rechargeable lithium batteries. Solid State Ionics 116:271–277
Rougier A, Saadoune I, Gravereau P, Willmannb P, Delmas C (1996) Effect of cobalt substitution on cationic distribution in LiNi1-yCoyO2 electrode materials. Solid State Ionics 90:83–90
Chen H, Dawson JA, Harding JH (2014) Effects of cationic substitution on structural defects in layered cathode materials LiNiO2. J Mater Chem A 2:7988–7996
Mukai K, Sugiyama J, Ikedo Y, Brewer JH, Ansaldo EJ, Morris GD, Ariyoshi K, Ohzuku T (2007) Microscopic magnetism in lithium insertion materials of LiNi1-xCoxO2(x = 0, 1/4, 1/2, 3/4, and 1). J Power Sources 174:843–846
Sekizawa O, Hasegawa T, Kitamura N, Idemoto Y (2011) Crystal and electronic structure change determined by various method for delithiation process of Lix(Ni, Mn)O2-based cathode material. J Power Sources 196:6651–6656
Pasero D, Reeves N, Gillie LJ, West AR (2007) Variable oxygen stoichiometry in layered rock salt cathodes, Lix(Mn, Ni)O2, depending on synthesis conditions. J Power Sources 174:1078–1081
Makimura Y, Ohzuku T (2003) Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium-ion batteries. J Power Sources 119–121:156–160
Delmas C, MeÂneÂtrier M, Croguennec L, Saadoune I, Rougier A, Pouillerie C, Prado G, GruÈne M, FourneÁs L (1999) An overview of the Li(Ni, M)O2 systems: syntheses, structures and properties. Electrochimica Acta 45:243–253
Julien C, Nazri GA, Rougier A (2000) Electrochemical performances of layered LiM1-yM1-y′O2 (M = Ni, Co; M′ = Mg, Al, B) oxides in lithium batteries. Solid State Ionics 135:121–130
Joeng JW, Kang SG (2003) Structural and electrochemical properties of LiNiyTi1-yO2 prepared by a wet process. J Power Sources 123:75–78
Zhang LQ, Noguchi H, Li DC, Muta T, Wang XQ, Yoshioa M, Taniguchi I (2008) Synthesis and electrochemistry of cubic rocksalt Li-Ni-Ti-O compounds in the phase diagram of LiNiO2-LiTiO2-Li[Li1/3Ti2/3]O2. J Power Sources 185:534–541
Nishida Y, Nakane K, Satoh T (1997) Synthesis and properties of gallium-doped LiNiO2 as the cathode material for lithium secondary batteries. J Power Sources 68:561–564
Koyama Y, Makimura Y, Tanaka I, Adachi H, Ohzuku T (2004) Systematic research on insertion materials based on superlattice models in a phase triangle of LiCoO2-LiNiO2-LiMnO2. J Electrochem Soc 151(9):A1499–A1506
Cao H, Xia BJ, Xu NX, Zhang CF (2004) Structural and electrochemical characteristics of Co and Al co-doped lithium nickelate cathode materials for lithium-ion batteries. J Alloy Compd 376:281–286
Bianchi V, Bach S, Belhomme C, Farcy J, Ramos JPP, Caurant D, Baffier N, Willmann P (2001) Electrochemical investigation of the Li insertion-extraction reaction as a function of lithium deficiency in Li1−xNi1+xO2. Electrochimica Acta 46:999–1011
Bianchi V, Caurant D, Baffier N, Belhomme C, Chappel E, Chouteau G, Bach S, Ramos JPP, Sulpice A, Wilmann P (2001) Synthesis, structural characterization and magnetic properties of quasistoichiometric LiNiO2. Solid State Ionics 140:1–7
Hwang S, Chang W, Kim SM, Su D, Kim DH, Lee JY, Chung KY, Stach AEA (2014) Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem Mater 26:1084–1092
Bak SM, Nam KW, Chang W, Yu XQ, Hu E, Hwang S, Stach EA, Kim KB, Chung KY, Yang AX (2013) Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem Mater 25:337–351
Watanabe S, Kinoshita M, Hosokawa T, Morigaki K, Nakura K (2014) Capacity fade of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests. J Power Sources 258:210–217
Cho Y, Cho J (2010) Significant improvement of LiNi0.8Co0.15Al0.05O2 cathodes at 60°C by SiO2 dry coating for Li-Ion batteries. J Electrochem Soc 157(6):A625–A629
Bi YJ, Yang WC, Du R, Zhou JJ, Liu M, Liu Y, Wang DY (2015) Correlation of oxygen non-stoichiometry to the instabilities and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 utilized in lithium ion battery. J Power Sources 283:211–218
Kosova NV, Devyatkina ET, Kaichev VV (2007) Optimization of Ni2 +/Ni3 + ratio in layered Li(Ni, Mn, Co)O2 cathodes for better electrochemistry. J Power Sources 174:965–969
Guilmard M, Pouillerie C, Croguennec L, Delmas C (2003) Structural and electrochemical properties of LiNi0.70Co0.15Al0.15O2. Solid State Ionics 160:39–50
Fu CC, Li GS, Luo D, Li Q, Fan JM, Li LP (2014) Nickel-rich layered microspheres cathodes: lithium/nickel disordering and electrochemical performance. ACS Appl Mater Interfaces 6:15822–15831
Yang HZ, Liu PX, Chen QL, Liu XW, Lu YW, Xie SF, Ni L, Wu XY, Peng MY, Chen YB, Tang YF, Chen YF (2014) Fabrication and characteristics of high-capacity LiNi0.8Co0.15Al0.05O2 with monodisperse yolk-shell spherical precursors by a facile method. RSC Advances 4:35522–35527
Wang ZY, Huang SS, Chen BJ, Wu H, Zhang Y (2014) Infiltrative coating of LiNi0.5Co0.2Mn0.3O2 microspheres with layer-structured LiTiO2 towards superior cycling performances for Li-ion batteries. J Mater Chem A 2(47):19983–19987
Lee KK, Kim KB (2000) Electrochemical and structural characterization of LiNi1–yCoyO2 (0 ≤ y ≤ 0.2) positive electrodes during initial cycling. J Electrochem Soc 147(5):1709–1717
Koyama Y, Tanaka I, Adachi H (2003) Crystal and electronic structures of superstructural Li1-x[Co1/3Ni1/3Mn1/3]O2 (0 ≤ x ≤ 1). J Power Sources 119–121:644–648
Abraham DP, Kawauchi S, Dees DW (2008) Modeling the impedance versus voltage characteristics of LiNi0.8Co0.15Al0.05O2. Electrochimica Acta 53:2121–2129
Hassoun J, Lee KS, Sun YK, Scrosati B (2011) An advanced Lithium Ion Battery based on high performance electrode materials. J Am Chem Soc 133:3139–3143
Arrebola BC, Caballero A, Cruz M, Hernán L, Morales J, Castellón ER (2006) Crystallinity control of a nanostructured LiNi0.5Mn1.5O4 spinel via polymer-assisted synthesis: a method for improving its rate capability and performance in 5 V lithium batteries. Adv Funct Mater 16:1904–1912
Caballero A, Hernan L, Melero M, Morales J, Angulo M (2005) Oxygen lattice instability as a capacity fading mechanism for 5 V cathode materials. J Electrochem Soc 152(1):A6–A12
Acknowledgements
The financial support of Creative fund of Chinese aerospace (2014-YF-0419) and China Postdoctoral Science Foundation (2012M520717) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruan, Z., Zhu, Y. & Teng, X. Effect of pre-thermal treatment on the lithium storage performance of LiNi0.8Co0.15Al0.05O2 . J Mater Sci 51, 1400–1408 (2016). https://doi.org/10.1007/s10853-015-9459-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9459-1