Abstract
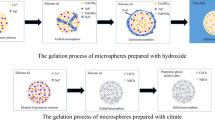
Calcium carbonate (CaCO3) is widely used as an important model system for investigating inorganic precipitation reaction or crystallization. However, recent results show that the yield of vaterite CaCO3 microspheres is poor in ethanol/water in the presence of polyelectrolyte poly(sodium 4-styrenesulfate) (PSS), which is up to 16 mM. We now report on an approach to synthesize pure vaterite CaCO3 microspheres through improving the concentration of polymer PSS and the yield is greatly high up to 80 mM. The exploration provides the possibility for large-scale synthesis of CaCO3 materials with controllable morphology and crystallographic structure in aqueous solution at room temperature. The possible formation mechanism toward the occurrence of vaterite CaCO3 microspheres has also been illustrated in virtue of a series of time-resolved experimental results. It is revealed that the vaterite microspheres evolve gradually from the initial amorphous precursor, to poorly crystallized nanoparticles, to sphere-like aggregates, and then to vaterite microspheres embedded with the calcite rhombohedra, finally to the vaterite microspheres with smooth surface. This research may provide a new viewpoint into the forming process of vaterite CaCO3 microspheres.








Similar content being viewed by others
References
Cölfen H, Qi L (2001) A Systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer. Chem Eur J 7:106–116
Preisig D, Haid D, Varum FJ et al (2014) Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur J Pharm Biopharm 87:548–558
Tang H, Yu JG, Ng DHL (2007) PSSS-controlled synthesis of CaCO3 superstructures. Cryst Res Technol 42:856–861
Mann S, Ozin GA (1996) Synthesis of inorganic materials with complex form. Nature 382:313–318
Yang H, Coombs N, Ozin GA (1997) Morphogenesis of shapes and surface patterns in mesoporous silica. Nature 386:692–695
Tang H, Yu JG, Zhao XF, Ng DHL (2008) Influence of PSSS on the morphology and polymorph of calcium carbonate in the ethanol–water mixed system. J Alloy Compd 463:343–349
Suda S, Ichikawa S, Wada N, Umegaki T (2000) Morphology of calcium carbonate coating on amorphous silicate powder. J Mater Sci 35:3023–3028. doi:10.1023/A:1004791129661
Zhao LN, Wang JK, Wang ZC (2013) Influnence of sodium polyacrylate on crystallization and aggregation of butterfly-like calcium carbonate. Chem Res Chin Univ 29:969–973
Zhao YY, Du W, Sun LM, Yu L, Jiao JJ, Wang R (2013) Facile synthesis of calcium carbonate with an absolutely pure crystal form using 1-butyl-3-methylimidazolium dodecyl sulfate as the modifier. Colloid Polym Sci 291:2191–2202
Guo YM, Wang FF, Zhang J et al (2013) Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids. Res Chem Intermed 39:2407–2415
Flaten EM, Seiersten M, Andreassen JP (2009) Polymorphism and morphology of calcium carbonate precipitated in mixed solvents of ethylene glycol and water. J Cryst Growth 311:3533–3538
Wang CY (2008) Control the polymorphism and morphology of calcium carbonate precipitation from a calcium acetate and urea solution. Mater Lett 62:2377–2380
Bao SP, Chen XY, Li Z, Yang BJ, Wu YC (2011) Effects of solvent and additive on controllable mineralization of MCO3 (M = Ca, Ba, Sr) crystals and their applications as red phosphors doped with Eu3+ ions. Cryst Eng Comm 13:2511–2520
Xu AW, Ma YR, Cölfen H (2007) Biomimetic mineralization. J Mater Chem 17:415–449
Mihai M, Mountrichas G, Pispas S et al (2013) Calcium carbonate microparticle templates using a PHOS-b-PMAA double hydrophilic copolymer. J Appl Crystallogr 46:1455–1466
Lei M, Tang WH, Cao LZ, Li PG, Yu JG (2006) Effects of poly (sodium 4-styrene-sulfonate) on morphology of calcium carbonate particles. J Cryst Growth 294:358–366
Yu JG, Tang H, Cheng B (2005) Influence of PSSS additive and temperature on morphology and phase structures of calcium oxalate. J Colloid Interf Sci 288:407–411
Meldrum F, Cölfen H (2008) Controlling mineral morphologies and structures in biological and synthetic systems. Chem Rev 108:4332–4432
Wang SS, Picker A, Cölfen H, Xu AW (2013) Heterostructured calcium carbonate microspheres with calcite equatorial loops and vaterite spherical cores. Angew Chem Int Ed 52:6317–6321
Chen HI, Chang HY (2004) Homogeneous precipitation of cerium dioxide nanoparticles in alcohol/water mixed solvents. Colloid Surf A 242:61–69
Dickinson SR, McGrath KM (2003) Switching between kinetic and thermodynamic control: calcium carbonate growth in the presence of a simple alcohol. J Mater Chem 13:928–933
Zhang L, Yue LH, Wang F, Wang Q (2008) Divisive effect of alcohol-water mixed solvents on growth morphology of calcium carbonate crystals. J Phys Chem B 112:10668–10674
Yao Y, Dong WY, Zhu SM, Yu XH, Yan DY (2009) Novel morphology of calcium carbonate controlled by poly(l-lysine). Langmuir 25:13238–13243
Smeets PJ, Cho KR, Kempen RG, Sommerdijk NA, De Yoreo JJ (2005) Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nat Mater 14:394–399
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Yang, B., Tang, H. et al. High-yield synthesis of vaterite CaCO3 microspheres in ethanol/water: structural characterization and formation mechanisms. J Mater Sci 50, 5540–5548 (2015). https://doi.org/10.1007/s10853-015-9101-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9101-2