Abstract
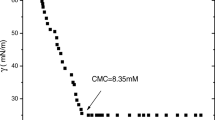
Cyclodextrins were found to play important roles in self-assembly systems of surfactants. The interactions between host molecule ß-cyclodextrin (CD) and model cationic surfactants, alkyltrimethylammonium bromides with different alkyl chain length: dodecyl-(C12TAB), tetradecyl-(C14TAB) and hexadecyl-(C16TAB) are studied by means of conductivity measurements at 313.2 K. The data obtained indicate that inclusion complexes (CD:S+) had formed, and apparent critical micelle concentration (CMC*) is equivalent to the combined concentrations of surfactant monomers complexed with the CD and that of a free dissolved monomer in equilibrium with the micellized surfactant without CD. Inclusion complexes were characterized by an equilibrium binding constant K 11, which value increases as the length of alkyl chains, and consequently the hydrophobicity, increases. From mathematical model the concentrations of the uncomplexed cyclodextrin, uncomplexed surfactant ion, and inclusion complex in the submicellar, as well as in the micellar range were calculated. The competition between the micellization and complexation processes leads to the existence of a significant concentration of free CD in equilibrium with the micellar aggregates. The percentage of uncomplexed cyclodextrin in equilibrium with the micelles is independent on cyclodextrin concentration for a particular ternary system and is 31, 37, and 34 % for C12TAB/water/ß-CD, C14TAB/water/ß-CD and C16TAB/water/ß-CD, respectively. By using standard Gibbs free energy for micellization and surfactant complexation by CD, we can explain the observed behavior.







Similar content being viewed by others
References
Szejtli, J.: Introduction and general overwiew of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Coleman, A.W., Nicolis, I., Keller, N., Dalbiez, J.P.: Aggregation of cyclodextrin-an explanation of the abnormal solubility of beta-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 13, 139–143 (1992)
Messner, M., Kurkov, S.V., Jansook, P., Loftsson, T.: Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 387, 199–208 (2010)
Valente, A.J.M., Soderman, O.: The formation of host-guest complexes between surfactants and cyclodextrins. Adv. Colloid Interface Sci. 205, 156–176 (2014)
Junquera, E., Aicart, E.: Potentiometric study of the encapsulation of ketoprophen by hydroxypropyl-β-cyclodextrin. Temperature, solvent, and salt effects. J. Phys. Chem. B 101, 7163–7171 (1997)
Kurkov, V.S., Loftsson, T.: Cyclodextrins. Int. J. Pharm. 453, 167–180 (2013)
Atkins, P., de Paula, J.: Physical Chemistry, 9th edn. W.H. Freeman and Company, New York (2010)
Fendler, J.H., Fendler, E.J.: Catalysis in micellar and macromolecular systems. Academic Press, New York (1975)
Marcolongo, J.P., Mirenda, M.: Thermodynamics of sodium dodecyl sulfate (SDS) micellization: an undergraduate laboratory experiment. J. Chem. Edu. 88, 629–633 (2011)
Badache, L., Lehanine, Z., Abderrahmane, W.A.: Synthesis and surface properties study of a series of cationic surfactants with different hydrophobic chain lengths. J. Surfact. Deterg. 15, 715–720 (2012)
Rosen, M.J., Kunjappu, J.T.: Surfactants and Interfacial Phenomena, 4th edn. Wiley, New Jersey (2012)
Jiang, L., Yan, Y., Huang, J.: Versatility of cyclodextrins in self-assembly systems of amphiphiles. Adv. Colloid Interface Sci. 169, 13–25 (2011)
Gaitano, Gonzalez-: G., Sanz-Garcia, T., Tardajos, G.: molar partial compressibilities and volumes, 1H NMR, and molecular modeling studies of the ternary systems β-cyclodextrin + sodium octanoate/sodium decanoate + water. Langmuir 15, 7963–7972 (1999)
Cabaleiro-Lago, C., Nilsson, M., Söderman, O.: Self-diffusion NMR studies of the host-guest interaction between β-cyclodextrin and alkyltrimethylammonium bromide surfactants. Langmuir 21, 11637–11644 (2005)
Valente, A.J.M., Dinis, C.J.S., Pereira, R.F.P., Ribeiro, A.C.F., Lobo, V.M.M.: Interactions between β-cyclodextrin and some sodium alkyl sulfates and sulfonates as seen by electrical conductivity measurements. Port. Electrochem. Acta 24, 129–136 (2006)
Li, S., Purdy, W.C.: Cyclodextrins and their applications in analytical-chemistry. Chem. Rev. 92, 1457–1470 (1992)
Astray, G., Gonzalez-Barreiro, C., Mejuto, J.C., Rial-Otero, R., Simal-Gándara, J.: A review on the use of cyclodextrins in foods. Food Hydrocolloids 23, 1631–1640 (2009)
Garcia-Rio, L., Leis, J.R., Mejuto, J.C., Pérez-Juste, J.: Investigation of micellar media containing β-cyclodextrin by means of reaction kinetics: basic hydrolysis of N-methyl-N-nitroso-p-toluenesulfonamide. J. Phys. Chem. B 101, 7383–7389 (1997)
Garcia-Rio, L., Leis, J.R., Mejuto, J.C., Pérez-Juste, J.: Basic hydrolysis of m-nitrophenyl acetate in micellar media containing β-cyclodextrin. J. Phys. Chem. B 102, 4581–4587 (1998)
Alvarez, A.R., Garcia-Rio, L., Hervés, P., Leis, J.R., Mejuto, J.C., Pérez-Juste, J.: Basic hydrolysis of substituted nitrophenyl acetates in β-cyclodextrin/surfactant mixed systems. Evidence of free cyclodextrin in equilibrium with micellized surfactant. Langmuir 15, 8368–8375 (1999)
Fernández, I., Garcia-Rio, L., Hervés, P., Mejuto, J.C., Pérez-Juste, J., Rodriguez-Dafonte, P.: β-cyclodextrin-micelle mixed systems as reaction medium. Denitrosation of N-methyl-N-nitroso-p-toluene-sulfonamide. J. Phys. Org. Chem. 13, 664–669 (2000)
Dharmawardana, U.R., Cristian, S.D., Tucker, E.E., Taylor, R.W., Scamchron, J.F.: A surface-tension method for determining binding constants for cyclodextrin inclusion complex of ionic surfactants. Langmuir 9, 2258–2263 (1993)
Tominaga, T., Hachisu, D., Kamado, M.: Interactions between the tetradecyltrimehylammonium ion and alpha-cyclodextrin, beta-cyclodextrin, and gamma-cyclodextrin in water as studied by a surfactant-selective electrode. Langmuir 10, 4676–4680 (1994)
Benko, M., Kiraly, Z.: Thermodynamics of inclusion complex formation of β-cyclodextrin with a variety of surfactants differing in the nature of head group. J. Chem. Thermodynamics 54, 211–216 (2012)
Qu, X.K., Zhu, L.Y., Li, L., Wei, X.L., Liu, F., Sun, D.Z.: Host-guest complexation of β-, γ-cyclodextrin with alkyl trimethyl ammonium bromides in aqueous solution. J. Solution Chem. 36, 643–650 (2007)
Junquera, E., Pena, L., Aicart, E.: A conductimetric study of the interaction of beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin with dodecyltrimethylammonium bromide in water solution. Langmuir 11, 4685–4690 (1995)
Junquera, E., Benito, J.G., Pena, L., Aicart, E.: Encapsulation processes of dodecyltrimethylammonium bromide into the β-cyclodextrin or 2,6-di-o-methyl-β -cyclodextrin cavities from speed of sound data. J. Colloid Interface Sci. 163, 355–361 (1994)
Lin, L.R., Jiang, Y.B., Du, X.Z., Huang, X.Z., Chen, G.Z.: A study of the properties of the 1:1 inclusion complex of β-cyclodextrin with cetyltrimethylammonium bromide. Chem. Phys. Lett. 266, 358–362 (1997)
Junquera, E., Pena, L., Aicart, E.: Micellar behavior of the aqueous solutions of dodecylethyldimethylammonium bromide. A characterization study in the presence and absence of hydroxypropyl-β-cyclodextrin. Langmuir 13, 219–224 (1997)
Mehta, S.K., Bhasin, K.K., Dham, S., Singla, M.L.: Micellar behavior of aqueous solutions of dodecyldimethylethylammonium bromide, dodecyltrimethylammonium chloride and tetradecyltrimethylammonium chloride in the presence of α-, β-, HPβ- and γ-cyclodextrins. J. Colloid Interface Sci. 321, 442–451 (2008)
Beiginejad, H., Bagheri, A.: Yekta Safdari L., Nojini, Z. B.: Thermodynamic studies of inclusion complex formation between alkylpyridinium chlorides and β-cyclodextrin using conductometric method. J. Incl. Phenom. Macrocycl. Chem. 67, 247–252 (2010)
Mwakibete, H., Bloor, D.M., Wyn-Jones, E.: Determination of the complexation constants between alkylpyridinium bromide and alpha- and beta-cyclodextrins using electromotive force methods. Langmuir 10, 3328–3331 (1994)
Sehgal, P., Sharma, M., Wimmer, R.: Larsen Lambertsen, K., Otzen, D. E.: Interactions between anionic mixed micelles and α-cyclodextrin and their inclusion complexes: conductivity, NMR and fluorescence study. Colloid Polym. Sci. 284, 916–926 (2006)
Dorrego, B., Garcia-Rio, L., Hervés, P., Leis, R.J., Mejuto, J.C., Pérez-Juste, J.: Changes in the fraction of uncomplexed cyclodextrin in equilibrium with the micellar system as a result of balance between micellization and cyclodextrin-surfactant complexation. J. Phys. Chem. B 105, 4912–4920 (2001)
Dorrego, A.B., Garcia-Rio, L., Hervés, P., Leis, J.R., Mejuto, J.C., Pérez-Juste, J.: Micellization versus cyclodextrin-surfactant complexation. Angew. Chem. Int. Ed. 39, 2945–2948 (2000)
Cabaliero-Lago, C., Garcia-Rio, L., Hervés, P., Mejuto, J.C., Pérez-Juste, J.: In search of fully uncomplexed cyclodextrin in the presence of micellar aggregates. J. Phys. Chem. B 110, 15831–15838 (2006)
Petek, A., Krajnc, M., Petek, A.: The role of intermolecular interactions in the micellization process of alkyltrimethylammonium bromides in water. Tenside Surf. Det. 53, 56–63 (2016)
Bai, Y., Xu, G.Y., Pang, J.Y., Sun, H.Y., Hao, A.Y., Xin, X., et al.: Comparative study on the effect of NaBr on the interaction between alkyltrimethylammonium bromide and β-cyclodextrin. J. Dispers. Sci. Technol. 31, 945–953 (2010)
Gonzalez-Gaitano, G., Crespo, A., Tardajos, G.: Thermodynamic investigation (volume and compressibility) of the systems β-cyclodextrin + n-alkyltrimethylammonium bromides + water. J. Phys. Chem. B 104, 1869–1879 (2000)
Guo, R., Zhu, X.J., Guo, X.: The effect of β-cyclodextrin on the properties of cetyltrimethylammonium bromide micelles. Colloid Polym. Sci. 281, 876–881 (2003)
Bakshi, M.S.: Cationic mixed micelles in the presence of β-cyclodextrin: a host-guest study. J. Colloid Interf. Sci. 227, 78–83 (2000)
Palepu, R., Reinsborough, V.C.: Surfactant-cyclodextrin interactions by conductance measurements. Can. J. Chem. 66, 325–328 (1988)
Jiang, B-y, Du, J., Cheng, S-q, Pan, J-w, et al.: Effects of cyclodextrins as additives on surfactant CMC. J. Disper. Sci. Technol. 24, 63–66 (2003)
Ghoreiski, S.M., Behpour, M., Golestaneh, M.: Study of inclusion complex formation between a cationic surfactant, two cyclodextrins and a drug. J. Incl. Phenom. Macrocycl. Chem. 62, 279–284 (2008)
Acknowledgments
Financial support by “The doctoral program is funded in part by the European Union through the European Social Fund. Co-financing is carried out within the framework of the Operational Programme Human Resources Development for the period 2007–2013, Development Priority 1, Promoting entrepreneurship and adaptability; policies priority_1. 3: Scholarship schemes. “ is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petek, A., Krajnc, M. & Petek, A. Study of host–guest interaction between ß-cyclodextrin and alkyltrimethylammonium bromides in water. J Incl Phenom Macrocycl Chem 86, 221–229 (2016). https://doi.org/10.1007/s10847-016-0656-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0656-6