Abstract
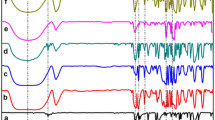
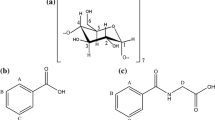
A series of positively charged β-cyclodextrin derivatives have been synthesized by selective functionalization of β-cyclodextrin at the primary rim with pyridinium groups. Characterisation of the modified β-cyclodextrin was done by elementary analysis, FTIR, 1H and 13C NMR spectroscopy. The inclusion complexation of adamantane derivatives (Adamantan-1-ol: AdOH and Sodium adamantane-1-carboxylate: AdCOO−Na+) by the host β-cyclodextrin and its grafted pyridinium derivatives has been investigated using 1H NMR spectroscopy. The stoichiometry of the complexes was found to be in 1:1 (adamantane:β-cyclodextrin) ratio. 1H chemical shift changes of adamantane protons were used to calculate the apparent binding constants of the complexes. Two dimentional NOESY experiments were performed to allow the mode of binding. Mono- and per-charged β-cyclodextrin showed an enhancement of inclusion binding ability towards the sodium adamantane-1-carboxylate guest. The origin of the observed enhancement in the stability of the complexes was ascribed to electrostatic interaction between carboxylate ion and charged pyridinium groups. A simple thermodynamic model of the electrostatic contribution to the complexation is presented.











Similar content being viewed by others
References
Szejtli, J.: Cyclodextrins and their inclusion complexes. Akademiai Kiado, Budapest (1982)
Loftsson, T., Duchêne, D.: Cyclodextrins and their pharmaceu-tical applications. Int. J. Pharm. 329, 1–11 (2007)
Sumit, S.V., Apeksha, K., Vasanti, S., Atul, P.S.: Influence of auxiliary agents on solubility and dissolution profile of repaglinide with hydroxypropyl-β-cyclodextrin: inclusion complex formation and its solid-state characterization. J. Incl. Phenom. Macrocycl. Chem. 83, 239–250 (2015)
Yutaka, I., Takashi, Y., Shota, W., Isamu, M., Ikuo, K.: Examination of intermolecular interaction as a result of cogrinding actarit and β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 78, 457–464 (2014)
Ogawa, N., Takahashi, C., Yamamoto, H.: Physicochemical characterization of cyclodextrin-drug interactions in the solid state and the effect of water on these interactions. J. Pharm. Sci. 104(3), 942–954 (2015)
Zhang, J.X., Ma, P.X.: Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug Deliv. Rev. 65(9), 1215–1233 (2013)
French, D., Levine, M.L., Pazur, J.H., Norberg, E.: Studies on the Schardinger dextrins. The preparation and solubility characteristics of α-, β- and γ-dextrins. J. Am. Chem. Soc. 71, 353–358 (1949)
Ashton, P.R., Boyd, S.E., Gattuso, G., Hartwell, E.Y., Koniger, R., Spencer, N., Stoddart, J.F.: A novel approach to the synthesis of some chemically-modified cyclodextrins. J. Org. Chem. 60, 3898–3903 (1995)
Wenz, G.: Cyclodextrins as building blocks for supramolecular structures and functional units. Angew. Chem. Int. Ed. Engl. 33, 803–822 (1994)
Zain, N.N.M., Raoov, M., Bakar, N.K.A., Mohamad, S.: Cyclodextrin modified ionic liquid material as a modifier for cloud point extraction of phenolic compounds using spectrophotometry. J. Incl. Phenom. Macrocycl. Chem. 84, 137–152 (2016)
Hbaieb, S., Kalfat, R., Chevalier, Y., Amdouni, N., Parrot-Lopez, H.: Influence of the substitution of β–cyclodextrins by cationic groups on the complexation of organic anions. Mater. Sci. Eng. C 28, 697–704 (2008)
Galaverna, G., Corradini, R., Dossena, A., Marcelli, R.: Histamine-modified cationic β-cyclodextrins as chiral selectors for the enantiomeric separation of hydroxy acids and carboxylic acids by capillary electrophoresis. Electrophoresis 20(13), 2619–2629 (1999)
Frbdkric, L., Carole, G., Pierre, G., Youssef, B., Hervé, G.: Use of a zwitterionic cyclodextrin as a chiral agent for the separation of enantiomers by capillary electrophoresis. Electrophoresis 18, 891–896 (1997)
Hansjorg, J., Markus, J., Volker, S.: Electrokinetic chromatography employing an anionic and a cationic β-cyclodextrin derivative. Electrophoresis 18, 897–904 (1997)
Abdul, R.K., Peter, F., Keith, J.S., Valerian, T.D.: Methods for selective modifications of cyclodextrins. Chem. Rev. 98, 1977–1996 (1998)
Zita, S., Ágnes, B.B., János, R.: pH-dependent complex formation of amino acids with β-cyclodextrin and quaternary ammonium β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 73, 199–210 (2012)
Martin, P., Simona, H., Jindřich, J.: A complete series of 6-deoxy-monosubstituted tetraalkylammonium derivatives of α-, β-, and γ-cyclodextrin with 1, 2, and 3 permanentpositive charges. Beilstein J. Org. Chem. 10, 1390–1396 (2014)
Daniel, G., Jorge, B., Maria, J.P., Mercedes, N., Wajih, A.: Host–guest complexation studied by fluorescence correlation spectroscopy: adamantane-cyclodextrin inclusion. Int. J. Mol. Sci. 11, 173–188 (2010)
Gao, J., Guo, Z.K., Su, F.J., Gao, L., Pang, X.H., Cao, W., Du, B., Wei, Q.: Ultrasensitive electrochemical immunoassay for CEA through host–guest interaction of β-cyclodextrin functionalized graphene and Cu@Ag core–shell nanoparticles with adamantinemodified antibody. Biosens. Bioelectron. 63(15), 465–471 (2015)
Shantanu, G.K., Zdenˇka, P., Michal, R., Lenka, D., Robert, V.: Adamantylated trisimidazolium-based tritopic guests and their binding properties towards cucurbit [7] uril and β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 84, 11–20 (2016)
Keivan, S., Ellen, E.M., Mark, W., Lee, J.: Association constant of β-cyclodextrin with carboranes, adamantane, and their derivatives using displacement binding technique. J. Incl. Phenom. Macrocycl. Chem. 83, 159–166 (2015)
John, S.W.: Medicinal properties of adamantane derivatives. J. Chem. Educ. 50(71), 780–781 (1973)
Agnieszka, L.C., Jerzy, S., Waclaw, K.: Comparative proton nuclear magnetic resonance studies of amantadine complexes formed in aqueous solutions with three major cyclodextrins. J. Pharm. Sci. 103, 274–282 (2014)
Harries, D., Rau, D.C., Parsegian, V.A.: Solutes probe hydration in specific association of cyclodextrin and adamantane. J. Am. Chem. Soc. 127, 2184–2190 (2005)
Ying-Ming, Z., Yong, C., Zhi-Qiang, L., Nan, L., Yu, L.: Quinolinotriazole-β-cyclodextrin and its adamantanecarboxylic acid complex as efficient water-soluble fluorescent Cd2+ sensors. Bioorg. Med. Chem. 18, 1415–1420 (2010)
Birgit, B., Lennart, K., Coine, S.M.: 1H-NMR Studies of the inclusion complexes betweena-cyclodextrin and adamantane derivatives using both exchangeable hydroxy protons and non-exchangeable aliphatic protons. J. Incl. Phenom. Macrocycl. Chem. 50, 173–181 (2004)
Yi-Che, S., Wan-Chun, C., Feng-Chih, C.: Preparation and characterization of polyseudorotaxanes based on adamantane-modified polybenzoxazines and β-cyclodextrin. Polymer 46, 1617–1623 (2005)
Baâzaoui, M., Béjaoui, I., Kalfat, R., Amdouni, N., Hbaieb, S., Chevalier, Y.: Preparation and characterization of nanoparticles made fromamphiphilic mono and per-aminoalkyl-β-cyclodextrins. Colloid Surf. A 484, 365–376 (2015)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Petter, R.C., Salek, J.S., Sikoski, C.T., Kumaravel, G., Lin, F.T.: Cooperative binding by aggregated mono-6-(alky1amino)-(β-cyclodextrins). J. Am. Chem. Soc. 112, 3860–3868 (1990)
Gadelle, A., Defaye, J.: Selective halogenation at primary positions of cyclomaltooligosaccharides and a synthesis of per-3,6-anhydro cyclomal-tooligosaccharides. Angew. Chem. Int. Ed. Engl. 30, 78–80 (1991)
Artur, J.M.V., Olle, S.: The formation of host–guest complexes between surfactants and cyclodextrins. Adv. Colloid Interface Sci. 205, 156–176 (2014)
Acknowledgments
This work was supported by a Grant from French–Tunisian cooperation project (CMCU n°04S1207).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Béjaoui, I., Baâzaoui, M., Chevalier, Y. et al. Influence of the substitution of β-cyclodextrins by pyridinium groups on the complexation of adamantane derivatives. J Incl Phenom Macrocycl Chem 86, 79–92 (2016). https://doi.org/10.1007/s10847-016-0643-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0643-y