Abstract
In the search of lead – free ferroelectric ceramics with improved properties, bismuth sodium titanate Na0.5Bi0.5TiO3 (NBT) was thoroughly investigated in our group. Solid solutions of the perovskite-type compounds, with Bi0.5(Na1-xLix)0.5TiO3, formula, were prepared by solid state reaction. The results of X-ray diffraction (XRD) data evidenced for a single perovskite phase, with a solubility limit of (Na,Li) estimated to x ≈ 0.2. The dielectric properties of sintered ceramics were investigated in wide temperature (25–800°C) and frequency (1kHz-1MHz) ranges. For low substitution rate, (0 ≤ x ≤ 0.2) the compounds exhibit diffuse phase transition and are characterised by high Curie temperature and a relaxor – like behavior. In the high temperature range, the thermal variation of permittivity is well described by a law \( \frac{1}{\varepsilon }-\frac{1}{\varepsilon_m}=C{\left(T-{T}_m\right)}^{\gamma } \), where γ is close to 1.5. Such a relaxor behavior is interpreted in terms of cation disorder, due to the statistical repartition of Na and Li, or correlated to the relaxation of polar clusters induced by the Na substitution by Li in the A site of the perovskite unit cell.
Similar content being viewed by others
1 Introduction
The bismuth sodium titanate compound Na0.5Bi0.5TiO3 (NBT) and NBT based solid solutions have attracted the attention of many workers. Indeed, it is considered to be a good piezoelectric - ferroelectric ceramic material, with its relatively large remnant polarization (Pr ≈ 40 μC/cm2) and high curie temperature (Tc = 320°C) [1, 2]. At room temperature, NBT is ferroelectric, with a rhombohedral perovskite-like structure (a = 0.388 nm, α = 89.8°) [3–6]. The Na+ and Bi3+ ions are randomly distributed at the 12 fold cuboctahedral sites [7]. NBT undergoes a series of temperature-induced phase transition: at ≈ 320°C, the symmetry changes from rhombohedral to tetragonal, and then into the cubic-one around 540°C [3, 8].
For a large number of ferroelectric perovskite compounds, the behavior can be modified by doping with appropriate cations [9–17]. Such modifications, when they involve cationic disorder they frequently produce relaxor-like materials, i.e., exhibiting a diffuse permittivity maximum which depends on frequency and temperature, so that they do not present a fully ordered phase transition at a strictly determined TC. This is associated to the space charge polarization induced by ions conductivity [11]. In addition, the permittivity of classical ferroelectrics follows the Curie-Weiss law above TC, while the relaxors deviate from it.
In the case of the NBT compound, the substitution of potassium for sodium lead to total solids solution having the formula Bi0.5(Na1-xKx)0.5TiO3, with 0 ≤ x ≤ 1. These compounds exhibit a relaxor-like behavior, probably induced by cationic disorder on the 12 fold cuboctahedral site of the perovskite lattice [12]. Similar results were given by Richard et al. [9] about NBT-ST system, NBT-BT solid solutions [9, 10] and Ba1-x(Bi0,5Li0,5)xTiO3 ceramics [15]. A double substitution of lithium and potassium for sodium was also previously reported, leading to solid-solutions of Bi0.5(Na1-x-yKxLiy)0.5TiO3 formula [18, 19]. The authors examined the solubility limits. They also studied the sintering conditions and the piezoelectric properties.
Up to now, the properties of Bi0.5(Na1-xLix)0.5TiO3 compounds have not yet been investigated. As the Li+ ion is smaller than Na+ (1.25 Å against 1.39 Å) [20], we can expect only a limited solubility of lithium in the Bi0.5(Na1-xLix)0.5TiO3 solid solutions. As a consequence, the aim of this article is to study the effects of the substitution of Li+ for Na+ on the dielectric properties of NBT based ceramics. In other terms, thermal and frequency evolutions of permittivity and dielectric loss are presented and discussed in terms of classical versus relaxor ferroelectric behavior.
2 Experimental procedure
Bi0.5(Na1-xLix)0.5TiO3 (0 ≤ x ≤0.2) solid solutions were prepared by conventional solid state reaction. Stoichiometric quantities of Bi2O3, TiO2, Na2CO3 and Li2CO3 (Aldrich > 99%) were thoroughly mixed for 3–4 h in an agate ball-miller, using acetone as liquid medium. Pure phases were obtained by calcination of the powders in air at 850°C for 12 h. After calcination, the ball-milled powders were uniaxially pressed (300 MPa) into discs of 10 mm in diameter and about 1–2 mm in thickness. Disk shaped ceramics, with densities close to 95% of theoretical, were obtained by conventional sintering at 1000–1200°C for 2–3 h in air. A subsequent annealing of all the materials was then performed at 950°C for a few hours. The microstructure of the sintered compacts was observed on etched surfaces using scanning electron microscope (Philips XL30).
In order to determine the symmetry of the samples and the limit of the solid solution, room temperature X-ray powder diffraction patterns were recorded using a θ/2θ diffractometer (Siemens D5000, Cu-Kα radiation, back monochromator), in the angular range 10 ≤ 2θ ≤ 90°. The evolution of cell parameters was examined via refinement of the peak positions. Dielectric measurements were performed on ceramic disks, after polishing and deposition of platinum electrode on the two circular faces of the pellets. Measurements were performed using an impedance analyser (HP 4194A), from room temperature to 800°C and between 100 Hz and 1 MHz.
3 Results and discussion
3.1 Solid solution limit and evolution of the lattice parameters
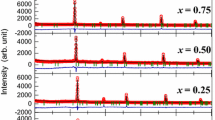
Figure 1 shows the XRD patterns of NBT-based materials. Preliminary examination of the raw X-ray powder diffraction data of the Bi0.5(Na1-xLix)0.5TiO3 samples for low Li content compositions (0 ≤ x ≤ 0.2 Fig. 1) suggested that these powders are single phase compounds and can be indexed with the rhombohedral symmetry of NBT. However, for x > 0.2, a secondary phase, assigned to Bi2Ti2O7- type, was always present. Consequently, the solubility limit can be estimated to be about x = 0.2.
The evolution of lattice parameters as a function of lithium content is given in Fig. 2. As it can be seen, the ″a″ lattice parameter decreases with the increasing x values. The distortion angle of the rhombohedral unit cell increases first, then it slightly decreases as the Li content increases. The unit cell volume (not represented here) also decreases, in a similar way as the ″a″ parameter.
This behavior was expected considering the respective sizes of the involved cations. In 12-fold coordination, the ionic radius of Na+ is 1.39 Å, against ≈ 1.25 Å (estimated value) for Li+ [20]. As a first consequence, the size of the pseudo-cubic perovskite cell must decrease, as experimentally observed. The corresponding tolerance factors, which constitute a rapid way to obtain informations about the stability of perovskite structures, can be estimated as being close to 1 for NBT (0.995) and 0.97 for the hypothetical compound Bi0.5Li0.5TiO3. As a second consequence, this compound, if existing, must probably have a different symmetry. And logically, we obtained a solubility limit (without structural change) for low Li content (10 mol%).
3.2 Dielectric measurements
The permittivity was measured as a function of temperature between 100 Hz and 1 MHz. For sake of legibility, the permittivity and loss data will be only given at five selected frequencies between 102, 103, 104, 105 and 106 Hz.
3.2.1 Pure NBT
Figure 3 shows the results obtained for unsubstituted NBT. As expected and in agreement with the literature, NBT undergoes a ferroelectric to paraelectric phase transition at 320°C [2, 3]. This is characterised by the sharp frequency independent maximum of ε’, associated to a minimum of tan δ. There is also a ″hump″ around 200°C, which is always observed for the NBT compound, and associated to a rapid change of the slope of the tan δ curve. At the present time, this phenomenon is not yet fully understood. It was sometimes assigned to a ferroelectric (rhombohedral)-antiferroelectric (tetragonal) phase transition [21, 22], but X-ray and neutron diffraction studies [3, 8] have revealed a complicated behavior, with coexistence of both rhomboedral and tetragonal symmetries between ≈ 250 and ≈ 400 °C. The structural analysis of the neutron diffraction data gave evidence for a rapid change of the Na/Bi cation shift around 200°C. As a consequence, the ″hump″ around this temperature is probably associated with a change in the local polar order.
For the above mentioned composition, the Curie constant C, calculated using the classical Curie-Weiss law \( {\varepsilon}^{-1} = \frac{T-\theta }{C} \) from the values of the permittivity- above the Curie point, is close to 6.105 K (Fig. 4). As expected for a first-order phase transition, the extrapolated Curie-Weiss temperature θ ≈ 165 °C is well below the Curie temperature (TC ≈ 325°C). In addition, the ratio of the slopes of the \( \frac{1}{\varepsilon }=f(T) \) curves, calculated before and after the phase transition (Fig. 4) is around 3.2, as the expected value is 4 for a first order phase transition (2 for a second order phase transition), using a Devonshire phenomenological analysis [23].
3.2.2 Bi0.5(Na1-xLix)0.5TiO3 solid solutions (0 ≤ x ≤0.2)
The situation is somewhat different for compositions where the lithium was introduced in the unit cell. The evolutions of the real and imaginary parts of the permittivity as a function of temperature and frequency for x = 0.05, 0.1, and 0.2 are given in Figs. 5, 6 and 7. The prominent features that can be observed are: (i) the hump is greatly enhanced, (ii) the phase transition (associated with the maximum of ε) is wide and shows some diffuse character, (iii) the temperature Tm of the maximum of the real permittivity (ε’m) increases as the measuring frequency increases, while the ε’m value decreases, (iv) the associated imaginary permittivity (ε”) exhibits a maximum at temperature close to the low temperature inflexion point of ε’. This maximum is also shifted towards the high temperature side as the frequency increases. After repeated heating and cooling experiments, this behavior remains almost unchanged. All these features are characteristic of the onset of a diffuse phase transition with relaxation process, i.e., a so-called “ferroelectric relaxor” behavior. Such behavior has already been mentioned for several KBT-NBT perovskite-type compounds [12, 24].
For temperatures higher than Tm, in these diffuse phase transition materials, the variation of the permittivity shows deviation from the classical Curie-Weiss law. Whatever the measurement frequency, the permittivity varies according to the law \( \frac{1}{\varepsilon }-\frac{1}{\varepsilon_m}=C{\left(T-{T}_m\right)}^{\gamma } \), where Tm is the temperature of the maximum permittivity εm at a given frequency, C is Curie like constant and γ is the degree of relaxation. The diffusion factor of γ is ranging from 1 for a normal ferroelectric to 2 for an ideal relaxor ferroelectric. This law (or similar ones) is often used to fit the permittivity data [25, 26]. Usually, the degree of relaxation γ is employed to describe the diffusion phase transition. The plots of Ln (\( \frac{1}{\varepsilon }-{\frac{1}{\varepsilon}}_m\Big) \) as a function of Ln(T – Tm) for the (Na1-x Lix)0.5Bi0.5TiO3 ceramics are shown in Fig. 8. It is noted that all samples exhibit a diffuse character of the phase transition. The results of such a fitting are collected in Table 1 and 2. The Curie constants are one to two orders of magnitude higher than those calculated for the weakly modified materials. The diffuseness of the phase transition is strongly supported by the value of γ, which changes from 1.6 to 1.82 as the substitution rate increases. The large difference observed in the temperatures of the maximum permittivities ΔT m = T m(HF) - T m(LF) at high and low frequencies (ΔTm increases as x increases) indicates that, for x ≥ 0.05, the ferroelectric relaxor behavior is more and more pronounced as x increases.
a Plots of Ln (\( \frac{1}{\varepsilon }-{\frac{1}{\varepsilon}}_m\Big) \) vs. Ln (T – Tm) for the (Na1-x Lix)0.5Bi0.5TiO3 ceramics at the frequency of 10 kHz. the symbols denote experimental data, while the solid lines denote the least squared fitting curves to the modified Curie Weiss law. b Variation of (\( \frac{1}{\varepsilon }-{\frac{1}{\varepsilon}}_m\Big) \) with (T – Tm) for various compositions in the (Na1-x Lix)0.5Bi0.5TiO3 system
Also the dielectric loss of (Na1-xLix)0.5Bi0.5TiO3 ceramics is influenced by Li addition remarkably. The unmodified material exhibit low dielectric loss characteristics in which the maximum of tanδ value is lower than 0.06. We note that substituted Li compounds show an increase in dielectric losses. In fact, the dielectric loss increase to 0.06 for x = 0 then to 1 for x = 0.1. Finally, it reaches 1.2 for x = 0.2. This is usually explained by an additional ionic conductivity due to the displacement of Li+ cations [15, 16].
Among the various models proposed to give an explanation of the relaxor behavior, the most probable that can apply for the materials under investigation would probably be the formation and the relaxation of dipoles or polar clusters, induced by the substitution. In the Bi0.5(Na1-xLix)0.5TiO3 solid solutions the charge distribution is strongly changed with respect to the initial NBT. As Na+, Li+ and Bi3+ are randomly distributed in the 12 fold coordination site, the relaxation–like behavior would be reasonably attributed to some disorder induced by the presence of Li+, as well as some Li+ or Na+ vacancies due to the sintering process. In addition, the possible distortions of the octahedral network depend rather on the nature of the 12-fold coordination cation than on the octahedral cation [7]. As a consequence, the diffuse character of the phase transition and the ferroelectric relaxor behavior could be connected with the increasing disorder of both Li+ and Na+ distributions.
4 Conclusion
Lithium modified bismuth sodium titanate Bi0.5(Na1-xLix)0.5TiO3 materials were successfully prepared as single phase by solid state reaction for 0 ≤ x ≤ 0.2 and conventionally sintered in air. For all compositions, densifications rates are in the range 92-99%. The dielectric study has shown that for low substitution rate, (0 ≤ x ≤ 0.2) the temperature of the maximum permittivity, for each frequency, increases significantly. We note also that the behavior of these materials changes from “normal” ferroelectric to relaxor type when x increases. The onset of relaxor behavior is probably correlated either to the relaxation of polar clusters induced by the substitution on A sites, or to cation disorder into the 12 fold coordination sites.
References
C. Wu, C. Yang, Cryst. Eng. Comm. 15, 9097 (2013)
Y. Li, R. Liao, X. Jiang, Y. Zhang, J. Alloys Compd. 484, 961 (2009)
G.O. Jones, P.A. Thomas, Acta Crys. B58, 168 (2002)
G.A. Smolenskii, V.A. Isupov, A.I. Agranoskaya, N.N. Krainik, Sov. Phys. Solid State. 2, 2651 (1961)
S.B. Vakhrushev, V.A. Isupov, B.E. Kvyatovsky, N.M. Okuneva, I.P. Pronin, Ferroelectrics 63, 153 (1985)
J.A. Zvirgzds, P.P. Kapostins, J.V. Zvirgzde, Ferroelectrics 40, 75 (1982)
M. Gröting, S. Hayn, K. Albe, J. Solid State Chem. 184, 2041 (2011)
J. Suchanicz, J. Kawpulinski, Ferroelectrics 165, 249 (1995)
J.R. Gomah-Pettry, S. Said, P. Marchet, J.P. Mercurio, J. Eur. Ceram. Soc. 24, 1165 (2004)
S. S. Sundari, B. Kumar, R. Dhanasekaran, IOP Conf. Series : Materials Science and Engineering, 43, (2013)
W. Lu, Y. Wang, G. Fan, X. Wang, F. Liang, J. Alloys Compd. 509, 2738 (2011)
S. Said, J.P. Mercurio, J. Eur. Ceram. Soc 21, 1333 (2001)
W.Z. Lu, G.F. Fan, X.H. Wang, F. Liang, Jpn. J. Appl. Phys. 450(11), 8763 (2006)
F. Gao, C.S. Zhang, X.C. Liu, L.H. Cheng et al., J. Eur. Ceram. Soc. 27, 3453 (2007)
M. Rawat, K.L. Yadav, Materials Chemistry and Physics,in press (2014)
J. Pozingis, J. Macutkevic, R. Grigalaitis, J. Banys, D.C. Lupascu, Ceram. Int. 40, 9961 (2014)
H.Y. Ma, X.M. Chen, J. Wang, K.T. Huo, H.L. Lian, P. Liu, Ceram. Int. 39, 3721 (2013)
D.M. Lin, L. Li, Mater. Lett. 58, 1333 (2004)
B. Ming, J. Wang, G. Zhang, Chin. Phys. Lett. 25(10), 3776 (2008)
R.D. Shannon, Acta Crystallogr. A32, 752 (1976)
K. Sakata, Y. Masuda, Ferroelectrics 7, 347 (1974)
T. Takenaka, K.I. Maruyama, K. Sakata, Japanese J. Appl; Phys. 20(9B), 2236 (1991)
Yuhuan Xu, Ferroelectric materials and their applications (North-Holland, 1991), chap.1
X. Yi, H. Chen, X. Cao, M. Zhao, D. Yang, G. Ma, C. Yang, J. Han, J. Cryst. Growth. 281, 364 (2005)
K. Uchino, S. Nomura, Ferroelectr. Lett. 44, 55 (1982)
S.M. Pilgrim, A.E. Sutherland, S.R. Winzer, J. Am. Ceram. Soc. 73, 3122 (1990)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Said, S., El Maaoui, M. Relaxor behavior in the Perovskitex-type Bi0.5(Na1-xLix)0.5TiO3 (0≤x≥0.2) solid solution. J Electroceram 35, 90–97 (2015). https://doi.org/10.1007/s10832-015-9996-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-015-9996-7