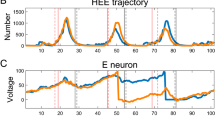
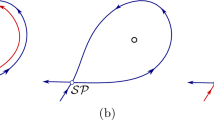
Abstract
The generation of intrinsic subthreshold (membrane potential) oscillations (STOs) in neuronal models requires the interaction between two processes: a relatively fast positive feedback that favors changes in voltage and a slower negative feedback that opposes these changes. These are provided by the so-called resonant and amplifying gating variables associated to the participating ionic currents. We investigate both the biophysical and dynamic mechanisms of generation of STOs and how their attributes (frequency and amplitude) depend on the model parameters for biophysical (conductance-based) models having qualitatively different types of resonant currents (activating and inactivating) and an amplifying current. Combinations of the same types of ionic currents (same models) in different parameter regimes give rise to different types of nonlinearities in the voltage equation: quasi-linear, parabolic-like and cubic-like. On the other hand, combinations of different types of ionic currents (different models) may give rise to the same type of nonlinearities. We examine how the attributes of the resulting STOs depend on the combined effect of these resonant and amplifying ionic processes, operating at different effective time scales, and the various types of nonlinearities. We find that, while some STO properties and attribute dependencies on the model parameters are determined by the specific combinations of ionic currents (biophysical properties), and are different for models with different such combinations, others are determined by the type of nonlinearities and are common for models with different types of ionic currents. Our results highlight the richness of STO behavior in single cells as the result of the various ways in which resonant and amplifying currents interact and affect the generation and termination of STOs as control parameters change. We make predictions that can be tested experimentally and are expected to contribute to the understanding of how rhythmic activity in neuronal networks emerge from the interplay of the intrinsic properties of the participating neurons and the network connectivity.















Similar content being viewed by others
References
Acker, C.D., Kopell, N., & White, J. A. (2003). Synchronization of strongly coupled excitatory neurons: relating network behavior to biophysics. Journal of Computational Neuroscience, 15, 71–90.
Alonso, A.A., & Klink, R. (1993). Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. Journal of Neurophysiology, 70, 144–157.
Alonso, A.A., & Llinás, R.R. (1989). Subthreshold Na+-dependent theta like rhythmicity in stellate cells of entorhinal cortex layer II. Nature, 342, 175–177.
Alonso, A., Khateb, A., Fort, P., Jones, B., & Mhlethaler, M. (1996). Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. The European Journal of Neuroscience, 8, 169–182.
Amir, R., Michaelis, M., & Devor, M. (1999). Membrane potential oscillations in dorsal root ganglion neurons role in normal electrogenesis and neuropathic pain. The Journal of Neuroscience, 19, 8589–8596.
Arvanitaki, A. (1939). Recherches sur la réponse oscillatoire locale de l’axone géant isolé de sepia. Archives Internationales de Physiologie, 49, 209–256.
Axmacher, N., Mormann, F., Fernandez, G., Elger, C.E., & Fell, J. (2006). Memory formation by neuronal synchronization. Brain Res. Rev., 170–1802.
Balu, R., Larimer, P., & Strowbridge, B.W. (2004). Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. Journal of Neurophysiology, 92, 743–753.
Baroni, F., Burkitt, A.N., & Grayden, D.B. (2014). Interplay of intrinsic and synaptic conductances in the generation of high-frequency oscillations in interneuronal networks with irregular spiking. PLoS Computational Biology, 10, e1003574.
Belluscio, M.A., Mizuseki, K., Schmidt, R., Kemper, R., & Buzsáki, G. (2012). Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. The Journal of Neuroscience, 32, 423–435.
Bilkey, D.K., & Heinemann, U. (1999). Intrinsic theta-frequency membrane potential oscillations in layer III/V perirhinal cortex neurons of the rat. Hippocampus, 9, 510–518.
Boehmer, G., Greffrath, W., Martin, E., & Hermann, S. (2000). Subthreshold oscillation of the membrane potential in magnocellular neurones of the rat supraoptic nucleus. The Journal of Physiology, 526, 115–128.
Bourdeau, M., Morin, F., Laurent, C., Azzi, M., & Lacaille, J. (2007). Kv4.3-mediated A-type K+ currentes underlie rhythmic activity in hippocampal interneurons. The Journal of Neuroscience, 27, 1942–1953.
Bracci, E., Centonze, D., Bernardi, G., & Calabresi, P. (2003). Voltage-dependent membrane potential oscillations in rat striatal fast-spiking interneurons. The Journal of Physiology, 15, 121– 130.
Bragin, A., Jando, G., Nadasdy, Z., Hetke, J., Wise, K., & Buzsáki, G. (1995). Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. The Journal of Neuroscience, 15, 47–60.
Brea, J.N., Kay, L.M., & Kopell, N.J. (2009). Biophysical model for gamma rhythms in the olfactory bulb via subthreshold oscillations. Proceedings of the National Academy of Sciences of the United States of America, 106, 21954–21959.
Burden, R.L., & Faires, J.D. (1980). Numerical analysis. Boston: PWS Publishing Company.
Chapman, C.A., & Lacaille, J.C. (1999). Intrinsic theta-frequency membrane potential oscillations in hippocampal CA1 interneurons of stratum lacunosum-moleculare. Journal of Neurophysiology, 81, 1296–1307.
Chorev, E., Yarom, Y., & Lampl, I. (2007). Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo. The Journal of Neuroscience, 27, 5043–5052.
Chrobak, J.J., & Buzsák, G. (1998). Gamma oscillations in the entorhinal cortex of the freely behaving rat. The Journal of Neuroscience, 18, 388–398.
Colgin, L. L. (2013). Mechanisms functions of theta rhythms. Annual Review of Neuroscience, 36, 295–312.
Colgin, L.L., Denninger, T., Fyhn, M., Hafting, T., Bonnevie, T., Jensen, O., Moser, M. -B., & Moser, E.I. (2009). Frequency of gamma oscillations routes flow of information in the hippocampus. Nature, 462, 353–358.
Cobb, S.R., Buhl, E.H., Halasy, K., Paulsen, O., & Somogyi, P. (1995). Synchronization of neuronal activity in the hippocampus by individual GABAergic interneurons. Nature, 378, 75–78.
Connors, B.W., & Amitai, Y (1997). Making waves in the neocortex. Neuron, 18, 347–349.
Desmaisons, D., Vincent, J.-D., & Lledo, P.-M. (1999). Control of action potential timing by intrinsic subthreshold oscillations in olfactory bulb output neurons. The Journal of Neuroscience, 19, 10727–10737.
Dickson, C.T., Magistretti, J., Shalinsky, M., Fransén, E., Hasselmo, M., & Alonso, A.A. (2000). Properties and role of I h in the pacing of subthreshold oscillation in entorhinal cortex layer II neurons. Journal of Neurophysiology, 83, 2562–2579.
Dickson, C.T., Magistretti, J., Shalinsky, M., Hamam, B., & Alonso, A.A. (2000). Oscillatory activity in entorhinal neurons and circuits. Annals of the New York Academy of Sciences, 911, 127–150.
Diekman, C.O., Belle, M.D.C., Irwin, R.P., Allen, C.N., Piggins, H.G., & Forger, D.B. (2013). Causes and consequences of hyperexcitation in central clock neurons. PLoS Computational Biology, 9, e1003196.
Dorval, A.D. Jr, & White, J.A. (2005). Channel noise is essential for perithreshold oscillations in entorhinal stellate neurons. The Journal of Neuroscience, 25, 10025–10028.
Dwyer, J., Lee, H., Martell, A., & van Drongelen, W. (2012). Resonance in neocortical neurons and networks. The European Journal of Neuroscience, 36, 3698–3708.
Fernandez, R., & White, J.A. (2008). Artificial synaptic conductances reduce subthreshold oscillations and periodic firing in stellate cells of the entorhinal cortex. The Journal of Neuroscience, 28, 3790–3803.
Fransén, E., Dickson, C.T., Magistretti, J., Alonso, A.A., & Hasselmo, M.E. (1998). Modeling the generation of subthreshold membrane potential oscillations of entorhinal cortex layer II stellate cells. Society for Neuroscience - Abstracts, 24, 814–815.
Fransén, E., Alonso, A.A., Dickson, C.T., Magistretti, M.E., & Hasselmo, J. (2004). Ionic mechanisms in the generation of subthreshold oscillations and action potential clustering in entorhinal layer II stellate neurons. Hippocampus, 14, 368–384.
Giocomo, L.M., Zilli, E.A., Fransén, E., & Hasselmo, M.E. (2007). Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science, 315, 1719–1722.
Gireesh, E., & Plenz, D. (2008). Neuronal avalanches organize as nested theta- and beta/gamma-oscillations during development of cortical layer 2/3. Proceedings of the National Academy of Sciences of the United States of America, 105, 7576–7581.
Gloveli, T., Dugladze, T., Rotstein, H.G., Traub, R., Monyer, H., Heinemann, U., Whittington, M.A., & Kopell, N. (2005). Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 102, 13295–13300.
Golomb, D. (2014). Mechanism and function of mixed-mode oscillations in vibrissa motoneurons. PLoS ONE, 9, e109205.
Golomb, D., Donner, K., Shacham, L., Shlosberg, D., Amitai, Y., & Hansel, D. (2007). Mechanisms of firing patterns in fast-spiking cortical interneurons. PLoS Computational Biology, 3, e156.
Gutfreund, Y., Yarom, Y., & Segev, I. (1995). Subthreshold oscillations and resonant frequency in guinea pig cortical neurons: physiology and modeling. The Journal of Physiology, 483, 621–640.
Harish, O., & Golomb, D. (2010). Control of the firing patterns of vibrissa motoneurons by modulatory and phasic synaptic inputs: a modeling study. Journal of Neurophysiology, 103, 2684– 2699.
Hinzer, K., & Longtin, A. (1996). Encoding with bursting, subthreshold oscillations, and noise in mammalian cold receptors. Neural Computation, 8, 215–255.
Hodgkin, A.L., & Huxley, A.F. (1952). A quantitative description of membrane current and its application to conductance and excitation in nerve. The Journal of Physiology, 117, 500–544.
Honeycutt, R.L. (1992). Stochastic runge-kutta algorithms. i. white noise. Physical Review A, 45, 600–603.
Hutcheon, B., & Yarom, Y. (2000). Resonance, oscillations and the intrinsic frequency preferences in neurons. Trends in Neurosciences, 23, 216–222.
Izhikevich, E.M. (2002). Resonance and selective communication via bursts in neurons having subthreshold oscillations. Bio Systems, 67, 95–102.
Izhikevich, E. (2006). Dynamical systems in neuroscience: the geometry of excitability and bursting. Cambridge: MIT Press.
Jalics, J., Krupa, M., & Rotstein, H.G. (2010). A novel mechanism for mixed-mode oscillations in a neuronal model. Dynamical Systems: an International Journal, 25, 445–482.
Jensen, O., & Colgin, L. L. (2007). Cross-frequency coupling between neuronal oscillations. Trends in Cognitive Sciences, 11, 267–269.
Kay, L.M., Beshel, J., Brea, J., Martin, C., Rojas-Líbano, D., & Kopell, N. (2008). Olfactory oscillations: the what, how and what for. Trends in Neurosciences, 32, 207–214.
Khosrovani, S., Van Der Giessen, R.S., De Zeeuw, C.I., & De Jeu, M.T.G. (2007). In vivo mouse inferior olive neurons exhibit heterogeneous subthreshold oscillations and spiking patterns. Proceedings of the National Academy of Sciences of the United States of America, 104, 15911–15916.
Kispersky, T., White, J.A., & Rotstein, H.G. (2008). The role of Kv7 mediated potassium currents and recurrent excitation in stellate cells of the entorhinal cortex in a dynamic clamp based model of temporal lobe epilepsy. Society for Neuroscience Abstracts, 250, 10.
Kispersky, T., White, J.A., & Rotstein, H.G. (2010). The mechanism of abrupt transition between theta and hyperexcitable spiking activity in medial entorhinal cortex layer II stellate cells. PloS One, 5, e13697.
Klink, R.M., & Alonso, A. (1993). Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. Journal of Neurophysiology, 70, 128–143.
Krupa, M., & Szmolyan, P. (2001). Extending geometric singular perturbation theory to nonhyperbolic points - fold and canard points in two dimensions. SIAM Journal on Mathematical Analysis, 33(2), 286–314.
Lampl, I, & Yarom, Y. (1993). Subthreshold oscillations of the membrane potential: a functional synchronizing and timing device. Journal of Neurophysiology, 70, 2181–2186.
Lampl, I, & Yarom, Y. (1997). Subthreshold oscillations and resonant behaviour: two manifestations of the same mechanism. Neuron, 78, 325–341.
Latorre, R., Aguirre, C., Rabinovich, M., & Varona, P. (2013). Transient dynamics and rhythm coordination of inferior olive spatio-temporal patterns. Frontier in Neural Circuits, 7, 138.
Latorre, R., Torres, J.J., & Varona, P. (2016). Interplay between subthreshold oscillations and depressing synapses in single neurons. PLoS ONE, 11, e0145830.
Llinás, R.R., & Yarom, Y. (1986). Oscillatory properties of guinea pig olivary neurons and their pharmachological modulation: an in vitro study. The Journal of Physiology, 376, 163–182.
Llinás, R.R., Grace, A.A., & Yarom, Y. (1991). In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-hz frequency range. Proceedings of the National Academy of Sciences of the United States of America, 88, 897–901.
Loewenstein, Y., Yarom, Y., & Sompolinsky, H. (2001). The generation of oscillations in networks of electrically coupled cells. Proceedings of the National Academy of Sciences of the United States of America, 98, 8095–8100.
Makarov, V.A., Nekorkin, V.I., & Velarde, M.G. (2001). Spiking behavior in a noise-driven system combining oscillatory and excitatory properties. Physical Review Letters, 15, 3031–3034.
Manis, P.B., Molitor, S.C., & Wu, H. (1999). Subthreshold oscillations generated by TTX-sensitive sodium currents in dorsal cochlear nucleus pyramidal cells. Experimental Brain Research, 153, 443–451.
Morin, F., Haufler, D., Skinner, F.K., & Lacaille, J.-C. (2010). Characterization of voltage-gated K+ currents contributing to subthreshold membrane potential oscillations in hippocampal CA1 interneurons. Journal of Neurophysiology, 103, 3472–3489.
McCarthy, M.M., Moore-Kochlacsa, C., Glub, X., Boyden, E.S., Han, X., & Kopell, N. (2011). Striatal origin of the pathologic beta oscillations in parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 108, 11620–11625.
Olypher, A.V., & Prinz, A.A. (2010). Geometry and dynamics of activity-dependent homeostatic regulation in neurons. Journal of Computational Neuroscience, 28, 361–374.
Pedroarena, C.M., Pose, I.E., Yamuy, J., Chase, M.H., & Morales, F.R. (1999). Oscillatory membrane potential activity in the soma of a primary afferent neuron. Journal of Neurophysiology, 82, 1465–1476.
Prinz, A.A., Thirumalai, V., & Marder, E. (2003). The functional consequences of changes in the strength and duration of synaptic inputs to oscillatory neurons. The Journal of Neuroscience, 23, 943–954.
Reboreda, A., Sanchez, E., Romero, M., & Lamas, J.A. (2003). Intrinsic spontaneous activity and subthreshold oscillations in neurones of the rat dorsal column nuclei in culture. The Journal of Physiology, 551, 191–205.
Remme, M.W.H., Lengyel, M., & Gutkin, B. S. (2012). A theoretical framework for the dynamics of multiple intrinsic oscillators in single neurons. In Schultheiss, N. W., Prinz, A.A., & Butera, R.A. (Eds.) Phase Response Curves in Neuroscience Theory, Experiments and Analysis (pp. 53–72): Springer.
Remme, M.W.H., Lengyel, M., & Gutkin, B.S. (2014). A trade-off between dendritic democracy and independence in neurons with intrinsic subthreshold membrane potential oscillations. In Cuntz, H., Remme, M.W.H., & Torben-Nielsen, B. (Eds.) The Computing Dendrite. New York: Springer.
Richardson, M.J.E., Brunel, N., & firing-rate, V. Hakim. (2003). From subthreshold to resonance. Journal of Neurophysiology, 89, 2538–2554.
Riecke, H., Roxin, A., Madruga, S., & Solla, S.A. (2007). Multiple attractors, long chaotic transients, and failure in small world networks of excitable neurons. Chaos, 17, 026110.
Rotstein, H.G. (2013). Abrupt and gradual transitions between low and hyperexcited firing frequencies in neuronal models with fast synaptic excitation: a comparative study. Chaos, 23, 046104.
Rotstein, H.G. (2014). Frequency preference response to oscillatory inputs in two-dimensional neural models: a geometric approach to subthreshold amplitude and phase resonance. The Journal of Mathematical Neuroscience, 4, 11.
Rotstein, H.G. (2015). Subthreshold amplitude and phase resonance in models of quadratic type: nonlinear effects generated by the interplay of resonant and amplifying currents. Journal of Computational Neuroscience, 38, 325–354.
Rotstein, H.G., & Nadim, F. (2014). Interaction between resonant and amplifying currents in two-dimensional neural models of frequency preference response to oscillatory input currents. Journal of Computational Neuroscience, 37, 9–28.
Rotstein, H.G., Oppermann, T., White, J.A., & Kopell, N. (2006). A reduced model for medial entorhinal cortex stellate cells: subthreshold oscillations, spiking and synchronization. Journal of Computational Neuroscience, 21, 271–292.
Rotstein, H.G., Wechselberger, M., & Kopell, N. (2008). Canard induced mixed-mode oscillations in a medial entorhinal cortex layer II stellate cell model. SIAM Journal on Applied Dynamical Systems, 7, 1582–1611.
Rotstein, H.G., Coombes, S., & Gheorghe, A. M. (2012). Canard-like explosion of limit cycles in two-dimensional piecewise-linear models of FitzHugh-Nagumo type. SIAM Journal on Applied Dynamical Systems, 11, 135–180.
Rotstein, H.G., Olarinre, M., & Golowasch, J. (2016). Dynamic compensation mechanism gives rise to period and duty cycle level sets in oscillatory neuronal models. Journal of Neurophysiology, 116(5), 2431–2452.
Roxin, A., Riecke, H., & Solla, S.A. (2004). Self-sustained activity in a small-world network of excitable neurons. Physical Review Letters, 92, 198101.
Sanhueza, M., & Bacigalupo, J. (2005). Intrinsic subthreshold oscillations of the membrane potential in pyramidal neurons of the olfactory amygdala. The European Journal of Neuroscience, 22, 1618–1626.
Serafin, M., Williams, S., Khateb, A., Fort, P., & Muhlethaler, M. (1996). Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience, 75, 671–675.
Schindewolf, C., Kim, D., Bel, A., & Rotstein, H. G. (2015). Complex patterns in networks of hyperexcitable neurons with multiple time scales. Theoretical Computer Science C, focus issue on Brain and Neural Networks Computing.
Schmitz, D., Gloveli, T., Behr, J., Dugladze, T., & Heinemann, U. (1998). Subthreshold membrane potential oscillations in neurons of deep layers of the entorhinal cortex. Neuron, 85, 999–1004.
Sharp, A.A., O’Neil, M.B., Abbott, L.F., & Marder, E. (1993). The dynamic clamp: artificial conductances in biological neurons. Trends in Neurosciences, 16, 389–394.
Skinner, F.K. (2006). Conductance-based models. Scholarpedia, 1, 1408.
Stiefel, K.M., Fellous, J.-M., Thomas, P.J., & Sejnowski, T.J. (2010). Intrinsic subthreshold oscillations extend the influence of inhibitory synaptic inputs on cortical pyramidal neurons. The European Journal of Neuroscience, 31, 1019–1026.
Tchumatchenko, T., & Clopath, C. (2014). Oscillations emerging from noise-driven steady state in networks with electrical synapses and subthreshold resonance. Nature Communications, 5, 5512.
Tikidji-Hamburyan, R.A., Martínez, J. J., White, J.A., & Canavier, C (2015). Resonant interneurons can increase robustness of gamma oscillations. The Journal of Neuroscience (in press), 35, 15682–15695.
Torben-Nielsen, B., Segev, I., & Yarom, Y. (2012). The generation of phase differences and frequency changes in a network model of inferior olive subthreshold oscillations. PLoS Computational Biology, 8, 31002580.
Wang, X.J. (1993). Ionic basis for intrinsic oscillations. Neuroreport, 5, 221–224.
Wang, X. J. (2002). Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. Journal of Neurophysiology, 87, 889–900.
Wang, X.J. (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Reviews, 90, 1195–1268.
White, J.A., Budde, T., & Kay, A.R. (1995). A bifurcation analysis of neuronal subthreshold oscillations. Biophysical Journal, 69, 1203–1217.
Wu, N., Hsiao, C.-F., & Chandler, S.H. (2001). Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: participants in burst generation. The Journal of Neuroscience, 21, 3729–3739.
Yoshida, M., & Alonso, A. (2007). Cell-type-specific modulation of intrinsic firing properties and subthreshold membrane oscillations by the m(Kv7)-currents in neurons of the entorhinal cortex. Journal of Neurophysiology, 98, 2779–2994.
Yoshida, M., Giocomo, L.M., Boardman, I., & Hasselmo, M.E. (2011). Frequency of subthreshold oscillations at different membrane potential voltages in neurons at different anatomical positions on the dorsoventral axis in the rat medial entorhinal cortex. The Journal of Neuroscience, 31, 12683–12694.
Zhuchkova, E., Remme, M.W.H., & Schreiber, S. (2014). Subthreshold resonance and membrane potential oscillations in a neuron with nonuniform active dendrite properties. In Cuntz, H., Remme, M.W.H., & Torben-Nielsen, B. (Eds.) The Computing Dendrite. New York: Springer.
Acknowledgments
This work was supported by the NSF grant DMS-1313861 (HGR). The author wishes to thank the anonymous reviewers for their detailed and thoughtful comments to the manuscript. This paper was partially written during the author’s visit to the Courant Institute of Mathematical Sciences at New York University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: J. Rinzel
Appendix A: Alternative linear model formulations
Appendix A: Alternative linear model formulations
1.1 A.1 Alternative dimensional formulation
The 3D system (4)–(6) can be viewed as a 2D system (4)–(5) coupled to the third variable ( w 2) whose dynamics is given by Eq. (6). This effective coupling depends not only on the linearized conductance g 2, but also on the time constant \( \bar {\tau }_{2} \). To capture this, it is useful to express system (4)–(6) in an alternative way by defining
By substituting into (4)-(5) and rearranging terms we obtain
where
Note that α, β and γ are dimensionless but system (14)–(16) is not. For g 2=0 ( α=1 and β=0), the 3D system reduces to the 2D system studied in Rotstein and Nadim (2014). The strength of the coupling between w 2 and this system depends on both g 2 and τ 2. Changes in g 2 and \( \bar {\tau }_{2} \) cause changes in both α and β. In particular, if g 2>0 ( g 2<0), corresponding to a resonant (amplifying) gating variable x 2, then α>1 ( α<1) and β>0 ( β<0).
1.2 A.2 Nondimensionalized linearized system
System (4)–(6) can be rescaled in order to reduce the number of parameters that effectively govern its dynamics (with no loss of information) and to uncover the effective strength of the terms in Eq. (4). We follow Richardson et al. (2003) and define the following dimensionless time and parameters
Substituting into (4)–(5) we obtain
Note that since v, w 1 and w 2 have the same units (of voltage) it is not necessary to rescale them (equivalently to rescale them by 1 mV).
1.3 Appendix B: Characterisic polynomial for 2D and 3D linearized systems
The characteristic polynomial for system (4)–(6) is given by
For the 2D linear system (4)–(5) with g 2=0, the characteristic polynomial reduces to
The roots of Eq. (23) are given by
and
Rights and permissions
About this article
Cite this article
Rotstein, H.G. The shaping of intrinsic membrane potential oscillations: positive/negative feedback, ionic resonance/amplification, nonlinearities and time scales. J Comput Neurosci 42, 133–166 (2017). https://doi.org/10.1007/s10827-016-0632-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-016-0632-6