Abstract
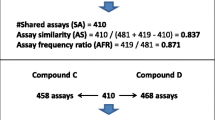
Activity cliffs are formed by pairs or groups of structurally similar compounds with significant differences in potency. They represent a prominent feature of activity landscapes of compound data sets and a primary source of structure–activity relationship (SAR) information. Thus far, activity cliffs have only been considered for active compounds, consistent with the principles of the activity landscape concept. However, from an SAR perspective, pairs formed by structurally similar active and inactive compounds should often also be informative. Therefore, we have extended the activity cliff concept to also take inactive compounds into consideration. As source of both confirmed active and inactive compounds, we have exclusively focused on PubChem confirmatory bioassays. Activity cliffs formed between pairs of active compounds (homogeneous pairs) and pairs of active and inactive compounds (heterogeneous pairs) were systematically analyzed on a per-assay basis, hence ensuring the currently highest possible degree of experimental consistency in activity measurement. Only very small numbers of large-magnitude activity cliffs formed between active compounds were detected in PubChem bioassays. However, when taking confirmed inactive compounds from confirmatory assays into account, the activity cliff frequency in assay data significantly increased, involving 11–15 % of all qualifying pairs of similar compounds, depending on the molecular representations that were used. Hence, these non-conventional activity cliffs provide an additional source of SAR information.





Similar content being viewed by others
References
Maggiora GM (2006) On outliers and activity cliffs—why QSAR often disappoints. J Chem Inf Model 46(4):1535
Stumpfe D, Bajorath J (2012) Exploring activity cliffs in medicinal chemistry. J Med Chem 55(7):2932–2942
Guha R, Van Drie JH (2008) Structure-activity landscape index: identifying and quantifying activity cliffs. J Chem Inf Model 48(3):646–658
Wassermann AM, Dimova D, Bajorath J (2011) Comprehensive analysis of single- and multi-target activity cliffs formed by currently available bioactive compounds. Chem Biol Drug Des 78(2):224–228
Wassermann AM, Wawer M, Bajorath J (2010) Activity landscape representations for structure—activity relationship analysis. J Med Chem 53(23):8209–8223
Medina-Franco JL, Martínez-Mayorga K, Bender A, Marín RM, Giulianotti MA, Pinilla C, Houghten RA (2009) Characterization of activity landscapes using 2D and 3D similarity methods: consensus activity cliffs. J Chem Inf Model 49(2):477–491
Hussain J, Rea C (2010) Computationally efficient algorithm to identify matched molecular pairs (MMPs) in large data sets. J Chem Inf Model 50(3):339–348
Hu X, Hu Y, Vogt M, Stumpfe D, Bajorath J (2012) MMP-cliffs: systematic identification of activity cliffs on the basis of matched molecular pairs. J Chem Inf Model 52(5):1138–1145
Hu Y, Bajorath J (2012) Extending the activity cliff concept: structural categorization of activity cliffs and systematic identification of different types of cliffs in the ChEMBL database. J Chem Inf Model 52(7):1806–1811
Seebeck B, Wagener M, Rarey M (2011) From activity cliffs to target-specific scoring models and pharmacophore hypotheses. ChemMedChem 6(9):1630–1639
Hu Y, Bajorath J (2012) Exploration of 3D activity cliffs on the basis of compound binding modes and comparison of 2D and 3D cliffs. J Chem Inf Model 52(3):670–677
Hu Y, Furtmann N, Gütschow M, Bajorath J (2012) Systematic identification and classification of three-dimensional activity cliffs. J Chem Inf Model 52(6):1490–1498
Berman H, Henrick K, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1):235–242
Vogt M, Huang Y, Bajorath J (2011) From activity cliffs to activity ridges: informative data structures for SAR analysis. J Chem Inf Model 51(8):1848–1856
Namasivayam V, Bajorath J (2012) Searching for coordinated activity cliffs using particle swarm optimization. J Chem Inf Model 52(4):927–934
Liu T, Lin Y, Wen X, Jorissen RN, Gilson MK (2007) BindingDB: a web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res 35(Database issue):D198–D201
Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP (2012) ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res 40(Database issue):D1100–D1107
Stumpfe D, Bajorath J (2012) Frequency of occurrence and potency range distribution of activity cliffs in bioactive compounds. J Chem Inf Model 52(9):2348–2353
Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Zhou Z, Han L, Karapetyan K, Dracheva S, Shoemaker BA, Bolton E, Gindulyte A, Bryant SH (2012) PubChem’s bioassay database. Nucleic Acids Res 40(Database issue):D400–D412
MACCS Structural Keys, Symyx Software: San Ramon, CA, 2005
Rogers D, Hahn M (2010) Extended-connectivity fingerprints. J Chem Inf Model 50(5):742–754
Willett P (2005) Searching techniques for databases of two- and three-dimensional structures. J Med Chem 48(13):4183–4199
Wawer M, Bajorath J (2010) Similarity-potency trees: a method to search for SAR information in compound data sets and derive SAR rules. J Chem Inf Model 50(8):1395–1409
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Maggiora, G.M. & Bajorath, J. Activity cliffs in PubChem confirmatory bioassays taking inactive compounds into account. J Comput Aided Mol Des 27, 115–124 (2013). https://doi.org/10.1007/s10822-012-9632-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9632-4