Abstract
Purpose
Polycystic ovary syndrome (PCOS) is the most common cause of ovulatory dysfunction and female infertility. The etiopathogenetic mechanisms of PCOS have been studied for many years, although exact causes remain unclear. It has been demonstrated that proteoglycan degradation by a disintegrin-like metalloproteinase with thrombospondin type motifs-1 (ADAMTS-1) is essential for ovulation and fertilization. The objective of our study is to analyze the levels of ADAMTS-1 and aggrecan in the follicular fluid (FF) of PCOS patients compared with normal ovulatory women and to determine whether these markers could be a predictor of in vitro fertilization (IVF) success in PCOS patients.
Methods
Women with PCOS (n = 21) and normal ovulatory controls (n = 22) undergoing IVF treatment were recruited. ADAMTS-1 and aggrecan levels were analyzed with enzyme-linked immunosorbent assay (ELISA) and compared between PCOS and normal ovulatory controls. The predictor effect of ADAMTS-1 and aggrecan on fertilization rate and implantation was evaluated.
Results
FF ADAMTS-1 and aggrecan levels increased in women with PCOS compared to controls. Elevated ADAMTS-1 levels but not aggrecan were related to increased implantation in PCOS.
Conclusion
Our study demonstrated that altered levels of ADAMTS-1 and aggrecan may have a partial role in the etiopathogenesis of PCOS, and ADAMTS-1 could be a predictive marker for implantation success in PCOS patients.
Similar content being viewed by others
Introduction
Polycystic ovary syndrome (PCOS) is a common and complex endocrine disorder affecting 5–10% of women of reproductive age [1]. It is associated with reproductive and metabolic abnormalities, such as menstrual irregularity, hyperandrogenism, infertility, and insulin resistance [2]. It is believed to impaired folliculogenesis, lack of development of a dominant follicle, and lack of ovulation in its pathophysiology [3].
Proteoglycans, the basic components of the extracellular matrix (ECM), consist of a protein core with a covalently attached glycosaminoglycan (GAG) side chain [4]. Human follicular fluid (FF) contains high concentrations of proteoglycan, which have important physiological effects on fertility. FF proteoglycans are synthesized by the granulosa cells at high levels [5]. Their synthesis is stimulated by follicle-stimulant hormone (FSH) and inhibited by luteinizing hormone (LH) and progesterone (PG) [6]. FF proteoglycans such as versican, aggrecan, and hyaluronan are important during folliculogenesis and antrum formation, because they increase the viscosity of FF due to their GAG chains [7] and form large aggregates [8]. Proteoglycans play an essential role in cumulus oocyte complex (COC) matrix expansion by combining with the oocyte at the time of ovulation. COC expansion provides the gel-like structure of the COC matrix, which protects the ovum on its passage towards the uterine cavity and facilitates ovum pick-up by increasing the chances for capture of the oocyte by the fimbriae. The proteoglycan aggrecan has been found mainly in the chondrocytes; however, several investigators have demonstrated its expression in various tissues, such as the endometrium [4].
Matrix metalloproteinases are involved in FF and the COC [9], and these proteinases regulate the degradation and regeneration of the ECM via a process called tissue remodeling. Extensive ECM remodeling of ovarian tissue is an essential and highly regulated event during folliculogenesis, ovulation, and fertilization in humans [10]. Various investigations have been conducted to determine the mechanisms involved in these proteolytic processes. However, the exact role of ECM remodeling in human reproduction has not been determined. A disintegrin-like and metalloproteinase with thrombospondin type motifs (ADAMTS)-1, forming a new family of metalloproteinases with catalytic activity against proteoglycans including versican and aggrecan [11], is essential during folliculogenesis, ovulation, and fertilization via ECM remodeling [10]. It is the only proteinase that has been well-established to affect ovulation [12]. An essential role of versican cleavage by ADAMTS-1 during ovulation and abnormal morphogenesis of versican in ADAMTS-1 null mice has been demonstrated [10]. ADAMTS-1 is mainly expressed in the granulosa cells of mammalian preovulatory follicles [13] and induced by LH via transactivation of the PG receptor [14]. Increasing levels in preovulatory follicles during ovulation has also been shown in nonhuman species [15]. Severely decreased fertility as a result of impaired follicular development and ovulation in ADAMTS-1 null species indicates its critical role in ovulation and folliculogenesis [13, 15]. ADAMTS-1 is also important in the remodeling of the COC matrix [16]. The hyaluronan-rich matrix assembles around ovulating oocytes by binding to versican for the expansion of COC [17]. It has also been demonstrated that the degradation of the COC matrix is necessary for sperm penetration, and it results in fertilization via versican cleavage by ADAMTS-1 [16]. However, there are limited studies about ADAMTS-1 expression and function in human ovulation, folliculogenesis, and fertilization.
The aim of our study was to examine FF aggrecan and ADAMTS-1 levels in developing and preovulatory follicles obtained from PCOS and normal ovulatory infertile patients undergoing in vitro fertilization (IVF) procedures. This study also aimed to investigate the etiopathogenetic mechanisms of PCOS and impaired fertility in PCOS patients, as well as the predictor effect of aggrecan and ADAMTS-1 on IVF outcomes, such as fertilization and implantation.
Materials and methods
The present study was a prospective clinical trial performed in the assisted reproduction clinic of a tertiary center between August 2016 and December 2016. A total of 43 patients undergoing IVF treatment—21 diagnosed as PCOS according to the Rotterdam consensus (the PCOS group) and 22 diagnosed as normal ovulatory women (the control group)—were recruited. Secondary causes of androgen excess and anovulation were excluded. The control group included women, who had received IVF for tubal factor and/or male infertility, except azoospermia and severe oligoasthenospermia with normal ultrasonographic ovarian morphology and regular ovulatory cycles.
Exclusion criteria were diminished ovarian reserve or endometriosis, systemic diseases, abnormal prolactin levels, and/or thyroid dysfunction. All participants were also free from tobacco, alcohol, and chronic medication use and major medical conditions. All women provided informed consent for participation. The procedures were conducted after approval from relevant local ethics committee.
Follicular fluid sampling
Patients were prepared for follicular sampling, using standard controlled ovarian stimulation with a gonadotropin-releasing hormone antagonist (cetrorelix) for down regulation of the pituitary gland. In all women, gonadotropin treatment was initiated on day 2 of the cycle according to the patient’s age, ovarian grade, and body mass index (BMI). Daily use of recombinant and/or urinary FSH was administered for ovarian stimulation. Ultrasonographic examination and blood sampling for estradiol (E2) levels were performed every 1–3 days. Gonadotropin doses were adjusted according to follicular development and E2 levels after the first 3–5 days of treatment. When the follicle diameter was 13 mm, patients received subcutaneous injections of 0.25 mg of cetrorelix. Oocyte retrieval was performed, using a 17-gauge oocyte aspiration needle 36 h after the administration of 250 μg of human chorionic gonadotropin (HCG) when at least three follicles that had reached 17 mm in diameter were detected. The collected oocytes were evaluated under invert microscopy to determine their developmental stage. The percentage of metaphase II (MII) oocytes was calculated by the following formula: (MII number/oocyte number) × 100.
The whole FF aspirates were centrifuged at 3000 rpm for 10 min to pellet the follicular cells. Only blood-free FF samples were stored at −80 °C until enzyme-linked immunosorbent assay (ELISA). On the same day, intracytoplasmic sperm injection (ICSI) of the oocytes was performed. Fertilization was evaluated 1 day later. Oocytes were considered successfully fertilized when two pronuclei were observed by light microscopy. The fertilization rate (FR) was calculated as the number of fertilized oocytes divided by the number of MII oocytes. Fresh intrauterine transfer of a single embryo with the highest quality at the eight-cell stage was performed on day 3 by a Wallace catheter. Implantation was determined by serum HCG levels performed 14 days after embryo transfer.
ADAMTS-1 and aggrecan assay in FF
Aggrecan levels were analyzed using human aggrecan ELISA kit (Cat. No: CK-E91941, Eastbiopharm, Hangzhou Eastbiopharm Co Ltd), and the results were expressed as nanograms per milliliter. The intra-assay and inter-assay coefficients of variability (CV) for aggrecan were <10 and <12%, respectively. The sensitivity was 0.09 ng/ml and the assay range was 0.2–60 ng/ml.
The ADAMTS-1 levels were detected through human ADAMTS-1 ELISA kit (Cat. No: CK-E90327, Eastbiopharm, Hangzhou Eastbiopharm Co Ltd), and values were presented as nanograms per milliliter. The intra-assay and inter-assay CV for ADAMTS-1 were <10 and <12%, respectively. The sensitivity was 0.25 ng/ml and the assay range was 0.5–30 ng/ml.
Statistical analyses
Data were analyzed using the SPSS version 17.0 statistical program. A p value <0.05 was considered as statistically significant. Continuous variables were tested for normality with the Kolmogorov–Smirnov test. Categorical comparisons were performed, using the chi-square test. We used independent samples t test and Mann-Whitney U test for parametric and nonparametric groups, respectively. Mean ± standard deviation (SD) was used in the descriptive statistics of data. The optimal cut-off points of aggrecan and ADAMTS-1 between PCOS and control groups were evaluated by receiver operating characteristic (ROC) analyses, calculating the area under the curve as giving the maximum sum of sensitivity and specificity. To determine whether aggrecan and ADAMTS-1 were independently associated with the FR, a linear regression analysis was performed, and a binary logistic regression analysis was used to determine the predictive value of aggrecan and ADAMTS-1 on implantation.
Results
Demographic features and aggrecan and ADAMTS-1 levels between PCOS and control groups
We divided patients undergoing IVF treatment into two groups: a PCOS group (diagnosed according to the Rotterdam criteria) and a control group (with normal ovulatory cycles). Age, partner’s age, and BMI were distributed homogeneously. FSH, LH, and PG levels at day 3 of the cycle and the percentage of MII were comparable across the groups with no statistically significant differences, whereas E2 levels were significantly higher in the PCOS group (p = 0.003) compared to controls. Oocyte, MII, and MI numbers were also higher in the PCOS group (p < 0.001, p = 0.003, and p = 0.011, respectively).
Follicular ADAMTS-1 levels were higher in the PCOS group (33.84 ± 17.7 ng/ml) than in controls (23.58 ± 5.67 ng/ml, p = 0.013). Aggrecan levels were also higher in women with PCOS (8.05 ± 4.78 ng/ml) compared to controls (3.63 ± 2.18 ng/ml, p < 0.001). Demographic features and the aggrecan and ADAMTS-1 levels in FF are shown in Table 1.
The cut-off level of ADAMTS-1 and aggrecan for the prediction of the diagnosis of PCOS
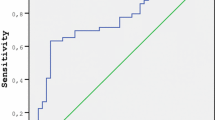
This study also determined the cut-off level, sensitivity, and specificity values of aggrecan and ADAMTS-1 for the prediction of the diagnosis of PCOS. Sensitivity was 72.7%, and specificity was 76.1% for aggrecan at a cut-off level of 4.10 ng/ml (p = 0.001). For ADAMTS-1, at a cut-off level of 27.50 ng/ml, the sensitivity was 72.2%, and the specificity was 68.0% (p = 0.013). ROC curve analyses considering sensitivity and specificity values are demonstrated in Fig. 1 and Table 2.
Predictive value of aggrecan and ADAMTS-1 on IVF outcome
To investigate the predictor effect of aggrecan and ADAMTS-1 on IVF outcomes, we evaluated FR after the ICSI procedure and implantation after embryo transfer. If an oocyte had two pronuclei or fragmented polar bodies, it was considered to have been fertilized. We calculated the FR as the number of fertilized oocytes divided by the number of MII oocytes. Results were classified as “low FR” if the FR was <50% and “normal FR” if the FR was ≥50%. Implantation was determined by serum HCG level measurements taken 14 days after the embryo transfer. A linear regression analysis failed to identify any predictor effect of aggrecan or ADAMTS-1 on FR in the PCOS group or among all patients (data not shown).
When implantation was evaluated, there was a statistically significant positive predictor effect of FF ADAMTS-1 (p = 0.036, β = 0.331) levels. However, there was no statistically significant effect of aggrecan on implantation via binary logistic regression analyses in PCOS women. Age, BMI, and the percentage of MII were not predictive of implantation (data not shown).
Discussion
Here, FF of PCOS and normal ovulatory women was obtained via IVF procedures to investigate ADAMTS-1 and aggrecan levels in the etiopathogenesis of PCOS. The results demonstrated elevated aggrecan and ADAMTS-1 levels in the FF of PCOS women, suggesting their potential role in the etiopathogenesis of PCOS. In contrast, Xiao et al. detected decreased expression levels of ADAMTS-1 in PCOS patients compared with normal ovulatory controls [12]. It has also been demonstrated that impaired follicular development and ovulation are present in ADAMTS-1 null mice [18]. In these studies, ADAMTS-1 level was evaluated in granulosa cells via polymerase chain reaction, whereas ADAMTS-1 was evaluated in FF by the ELISA method in our study. Therefore, the results could have been affected by the species and the evaluation method. A 40% reduction in follicle numbers in ADAMTS-1 null mice indicates that ECM remodeling by ADAMTS-1 is necessary for folliculogenesis in mice [13]. In our study, MII and MI oocyte numbers were higher in PCOS patients. Hence, increased numbers of MII and MI oocytes in the PCOS group could have caused elevated ADAMTS-1 levels in these women.
Although proteoglycans were investigated in the etiopathogenesis of follicular maturation and anovulation, ours is the first study evaluating FF aggrecan levels in PCOS patients. Our data suggest a potential role of aggrecan in the etiopathogenesis of PCOS. Lack of versican cleavage in the basal region of the follicle in ADAMTS-1 null mutant has been shown to be associated with reduced ovulation rates [10]. A previous study also confirmed a negative association between follicular size and the concentration of GAG in FF [19], suggesting the critical role of GAGs in follicular maturation.
The results of our study did not show a significant correlation between FR and follicular ADAMTS-1 and aggrecan levels. One limitation of our study was the lack of evaluation of oocyte quality, which is closely associated with fertilization. It has been shown that decreased ADAMTS-1 levels are closely related to lower oocyte fertilization capacity [12]. Yung et al. demonstrated upregulation of ADAMTS-1 expression during follicular maturation and elevated ADAMTS-1 levels in cumulus cells from fertilized oocytes, suggesting that ADAMTS-1 could serve as a marker for fertilization capacity [18]. Reduced ovulation and FR in ADAMTS-1 null mice and abnormal morphogenesis of versican and hyaluronan in the basal region of follicles have been demonstrated previously [10]. A negative correlation between the follicular chondroitin sulfate (CS) concentration, a type of glycosaminoglycan, and FR has also been demonstrated [19]. Bellin et al. also reported that lower and higher concentrations of CS were associated with lower FR but were unrelated to the quality of the embryos, whereas high concentrations of heparan sulfate were associated with higher-quality embryos but were unrelated to the FR [20]. Brown et al. also reported reduced rates of fertilization in vivo but not in vitro in ADAMTS-1 null mice, which is similar to the results of our study [10]. Reduced rate of in vivo fertilization could be a result of inhibited sperm penetration via reduced clearance of versican from the matrix [10]. However, in vitro FR is less dependent on the COC matrix function and influenced primarily by medium conditions.
To the best of our knowledge, there are no studies evaluating the correlation between follicular ADAMTS-1 and aggrecan levels and implantation. A positive predictor effect of ADAMTS-1 (but not aggrecan) on implantation in our study indicates that follicular ADAMTS-1 levels could be a potential marker of high-quality embryos for transfer in PCOS patients.
Conclusion
In conclusion, increase of ADAMTS-1 and aggrecan in the FF may have a partial role in the etiopathogenesis of PCOS and follicular ADAMTS-1 is a potential predictor marker for implantation capacity in PCOS patients. Further studies are needed to investigate the etiopathogenetic factors of PCOS and to improve IVF success rates in PCOS patients.
References
Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:304–11.
Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28:2562–9.
Corbett S, Morin-Papunen L. The polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol. 2013;373:39–50.
Yasuo T, Yamaguchi T, Kitaya K. Progesterone induction of chondroitin sulfate proteoglycan aggrecan expression in human endometrial epithelial cells. J Steroid Biochem Mol Biol. 2010;122:159–63.
Yanagishita M, Hascall VC. Biosynthesis of proteoglycans by rat granulosa cells cultured in vitro. J Biol Chem. 1979;254:12355–64.
Mueller PL, Schreiber JR, Lucky AW, Schulman JD, Rodbard D, Ross GT. Follicle-stimulating hormone stimulates ovarian synthesis of proteoglycans in the estrogen-stimulated hypophysectomized immature female rat. Endocrinology. 1978;102:824–31.
Yanagishita M. Proteoglycans and hyaluronan in female reproductive organs. EXS. 1994;70:179–90.
Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta. 1812;2011:1616–29.
Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312.
Brown HM, Dunning KM, Robker RL, Boerboom D, Pritchard M, Lane M, et al. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83:549–57.
Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–8.
Xiao S, Li Y, Chen M, Xu Y, Wen Y, Zhou C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J Clin Endocrinol Metab. 2014;99:1015–21.
Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709.
Doyle KM, Russell DL, Sriraman V, Richards JS. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol Endocrinol. 2004;18:2463–78.
Boerboom D, Russell DL, Richards JS, Sirois J. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin-like motifs-1) and progesterone receptor by human chorionic gonadotropin in equine preovulatory follicles. J Mol Endocrinol. 2003;31:473–85.
Russell, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144:1020–31.
Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–27.
Yung Y, Maman E, Konopnicki S, Cohen B, Brengauz M, Lojkin I, et al. ADAMTS-1: a new human ovulatory gene and a cumulus marker for fertilization capacity. Mol Cell Endocrinol. 2010;328:104–8.
Eriksen GV, Malmstrom A, Uldbjerg N. Human follicular fluid proteoglycans in relation to in vitro fertilization. Fertil Steril. 1997;68(5):791–8.
Bellin ME, Ax RL, Laufer N, Tarlatzis BC, DeCherney AH, Feldberg D, et al. Glycosaminoglycans in follicular fluid from women undergoing in vitro fertilization and their relationship to cumulus expansion, fertilization, and development. Fertil Steril. 1986;45:244–8.
Acknowledgements
E.N.T. formulated the present hypothesis and was responsible for writing the report. D.U.K. and B.O. collected the data and M.E. was responsible for analyzing the data. N.K. was responsible for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tola, E.N., Karatopuk, D.U., Koroglu, N. et al. Follicular ADAMTS-1 and aggrecan levels in polycystic ovary syndrome. J Assist Reprod Genet 34, 811–816 (2017). https://doi.org/10.1007/s10815-017-0913-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0913-7