Abstract
Purpose
In an attempt to improve in vitro embryo production, we investigated the effect of fibroblast growth factor 10 (FGF10) during in vitro maturation on the developmental capacity of bovine oocytes.
Material and methods
Cumulus–oocyte complexes (COCs) were aspirated from follicles of 3–8 mm diameter. After selection, the COCs were matured in medium with or without 0.5 ng/mL of FGF10. The effect of FGF10 during in vitro maturation (IVM) on nuclear maturation kinetics and expansion of the cumulus cells was investigated. Oocyte competence was assessed by the production and development speed of embryos and the relative expression of genes associated with embryo quality.
Results
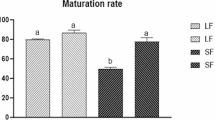
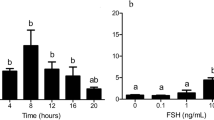
FGF10 delayed the resumption of meiosis from 8 h onwards, but did not affect the percentage of oocytes reaching metaphase II, nor did it increase cumulus expansion at 22 h of maturation. We found no difference between treatments regarding embryo production, developmental speed, and gene expression.
Conclusion
In conclusion, the presence of FGF10 during IVM had no effect on embryo production, developmental speed, and gene expression.



Similar content being viewed by others
References
Webb R et al. Intra-ovarian regulation of follicular development and oocyte competence in farm animals. Theriogenology. 2007;68 Suppl 1:S22–9.
Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26(1):63–77.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–66.
Gasperin BG et al. FGF10 inhibits dominant follicle growth and estradiol secretion in vivo in cattle. Reproduction. 2012;143(6):815–23.
Castilho AC et al. Expression of fibroblast growth factor 10 and cognate receptors in the developing bovine ovary. Theriogenology. 2014;81(9):1268–74.
Portela VM et al. The role of fibroblast growth factor-18 in follicular atresia in cattle. Biol Reprod. 2015;92(1):14.
Cho JH et al. Fibroblast growth factor 7 stimulates in vitro growth of oocytes originating from bovine early antral follicles. Mol Reprod Dev. 2008;75(12):1736–43.
Price CA. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2016;228(2):R31–43.
Taniguchi F et al. Aberrant expression of keratinocyte growth factor receptor in ovarian surface epithelial cells of endometrioma. Fertil Steril. 2008;89(2):478–80.
Oron G et al. Fibroblast growth factor 10 in human ovaries. Reprod Biomed Online. 2012;25(4):396–401.
Buratini Jr J et al. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 2B, in bovine follicles. Biol Reprod. 2007;77(4):743–50.
Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction. 2010;140(6):815–26.
Zhang K, Ealy AD. Disruption of fibroblast growth factor receptor signaling in bovine cumulus-oocyte complexes during in vitro maturation reduces subsequent embryonic development. Domest Anim Endocrinol. 2012;42(4):230–8.
Caixeta ES et al. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction. 2013;146(1):27–35.
Pomini Pinto RF et al. Effects of FGF10 on bovine oocyte meiosis progression, apoptosis, embryo development and relative abundance of developmentally important genes in vitro. Reprod Domest Anim. 2015;50(1):84–90.
Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995;44(6):859–69.
Machado GM et al. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009;71(8):1289–97.
Holm P et al. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology. 1998;50(8):1285–99.
Vandesompele J et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45.
Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139–49.
Buchtova M et al. Instability restricts signaling of multiple fibroblast growth factors. Cell Mol Life Sci. 2015;72(12):2445–59.
Liang CG et al. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21(9):2037–55.
Salhab M et al. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80(2):166–82.
Tomek W, Smiljakovic T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction. 2005;130(4):423–30.
Ali A, Sirard MA. Effect of the absence or presence of various protein supplements on further development of bovine oocytes during in vitro maturation. Biol Reprod. 2002;66(4):901–5.
Ali A, Sirard MA. The effects of 17beta-estradiol and protein supplement on the response to purified and recombinant follicle stimulating hormone in bovine oocytes. Zygote. 2002;10(1):65–71.
Ghanem N et al. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction. 2011;142(4):551–64.
Machado GM et al. Post-hatching development of in vitro bovine embryos from day 7 to 14 in vivo versus in vitro. Mol Reprod Dev. 2013;80(11):936–47.
El-Halawany N et al. Quantitative expression analysis of blastocyst-derived gene transcripts in preimplantation developmental stages of in vitro-produced bovine embryos using real-time polymerase chain reaction technology. Reprod Fertil Dev. 2005;16(8):753–62.
El-Sayed A et al. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol Genomics. 2006;28(1):84–96.
Wrenzycki C, Herrmann D, Niemann H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology. 2007;68:S77–83.
Hoelker M et al. Molecular signatures of bovine embryo developmental competence. Reprod Fertil Dev. 2013;26(1):22–36.
Loren, P., et al. Effect of short-term exposure of cumulus–oocyte complex to 3-morpholinosydnonimine on in vitro embryo development and gene expression in cattle. Reproduction in Domestic Animals. 2016; 51(6):1010–19.
Jackson BW et al. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–79.
Machado GM et al. Post-hatching development of bovine embryos in vitro: the effects of tunnel preparation and gender. Zygote. 2012;20(2):123–34.
Skouri-Panet F et al. Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: role of the in vitro hetero-complex formation in chaperone activity. Biochimie. 2012;94(4):975–84.
Habraken Y et al. Binding of insertion/deletion DNA mismatches by the heterodimer of yeast mismatch repair proteins MSH2 and MSH3. Curr Biol. 1996;6(9):1185–7.
Galaviz-Hernandez C et al. Plac8 and Plac9, novel placental-enriched genes identified through microarray analysis. Gene. 2003;309(2):81–9.
Touzard E et al. Specific expression patterns and cell distribution of ancient and modern PAG in bovine placenta during pregnancy. Reproduction. 2013;146(4):347–62.
Green JA et al. The establishment of an ELISA for the detection of pregnancy-associated glycoproteins (PAGs) in the serum of pregnant cows and heifers. Theriogenology. 2005;63(5):1481–503.
Xiang W, MacLaren LA. Expression of fertilin and CD9 in bovine trophoblast and endometrium during implantation. Biol Reprod. 2002;66(6):1790–6.
Acknowledgements
The authors thank the Brazilian Agricultural Research Corporation (EMBRAPA, 01.13.06.001.04.00) and Coordination for Improvement of Higher Education Personnel (CAPES, 564376/2010-8) for their financial support and the Qualimax (Luziânia-GO) slaughterhouse for providing the necessary biological materials for this experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Use of FGF 10 during IVM of bovine oocytes does not affect embryonic development.
Rights and permissions
About this article
Cite this article
Diógenes, M.N., Guimarães, A.L.S., Leme, L.O. et al. Bovine in vitro embryo production: the effects of fibroblast growth factor 10 (FGF10). J Assist Reprod Genet 34, 383–390 (2017). https://doi.org/10.1007/s10815-016-0852-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0852-8