Abstract
Purpose
Temporary and reversible downregulation of metabolism may improve the survival of tissues exposed to non-physiological conditions during transport, in vitro culture, and cryopreservation. The objectives of the study were to (1) optimize the concentration and duration of carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP—a mitochondrial uncoupling agent) exposures for biopsies of domestic cat ovarian tissue and (2) examine the effects of FCCP pre-exposures on follicle integrity after tissue culture and/or cryopreservation.
Methods
Biopsies of cat ovarian tissue were first treated with various concentrations of FCCP (0, 10, 40, or 200 nM) for 10 or 120 min to determine the most suitable pre-exposure conditions. Based on these results, tissues were pre-exposed to 200 nM FCCP for 120 min for the subsequent studies on culture and cryopreservation. In all experiments and for each treatment group, tissue activity and integrity were measured by mitochondrial membrane potential (relative optical density of rhodamine 123 fluorescence), follicular viability (calcein assay), follicular morphology (histology), granulosa cell proliferation (Ki-67 immunostaining), and follicular density.
Results
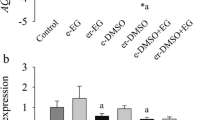
Ovarian tissues incubated with 200 nM FCCP for 120 min led to the lowest mitochondrial activity (1.17 ± 0.09; P < 0.05) compared to control group (0 nM; 1.30 ± 0.12) while maintaining a constant percentage of viable follicles (75.3 ± 7.8 %) similar to the control group (71.8 ± 11.7 %; P > 0.05). After 2 days of in vitro culture, percentage of viable follicles (78.8 ± 8.9 %) in similar pre-exposure conditions was higher (P < 0.05) than in the absence of FCCP (61.2 ± 12.0 %) with percentages of morphologically normal follicles (57.6 ± 17.3 %) not different from the fresh tissue (70.2 ± 7.1 %; P > 0.05). Interestingly, percentages of cellular proliferation and follicular density were unaltered by the FCCP exposures. Based on the indicators mentioned above, the FCCP-treated tissue fragments did not have a better follicle integrity after freezing and thawing.
Conclusions
Pre-exposure to 200 nM FCCP during 120 min protects and enhances the follicle integrity in cat ovarian tissue during short-term in vitro culture. However, FCCP does not appear to exert a beneficial or detrimental effect during ovarian tissue cryopreservation.





Similar content being viewed by others
References
Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13.
Wiedemann C, Hribal R, Ringleb J, Bertelsen MF, Rasmusen K, Andersen CY, et al. Preservation of primordial follicles from lions by slow freezing and xenotransplantation of ovarian cortex into an immunodeficient mouse. Reprod Domest Anim. 2012;47 Suppl 6:300–4.
Jewgenow K, Paris MC. Preservation of female germ cells from ovaries of cat species. Theriogenology. 2006;66:93–100.
Songsasen N, Comizzoli P, Nagashima J, Fujihara M, Wildt DE. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod Domestic Anim = Zuchthygiene. 2012;47:13–8.
Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility for females of wild and endangered mammals. Cancer Treat Res. 2010;156:87–100.
Wiedemann C, Zahmel J, Jewgenow K. Short-term culture of ovarian cortex pieces to assess the cryopreservation outcome in wild felids for genome conservation. BMC Vet Res. 2013;9:37.
Higuchi CM, Maeda Y, Horiuchi T, Yamazaki Y. A simplified method for three-dimensional (3-D) ovarian tissue culture yielding oocytes competent to produce full-term offspring in mice. PLoS One. 2015;10:e0143114.
Thuwanut P, Chatdarong K. Cryopreservation of cat testicular tissues: effects of storage temperature, freezing protocols and cryoprotective agents. Reprod Domest Anim. 2012;47:777–81.
Cleary M, Snow M, Paris M, Shaw J, Cox SL, Jenkin G. Cryopreservation of mouse ovarian tissue following prolonged exposure to an Ischemic environment. Cryobiology. 2001;42:121–33.
Evecen M, Cirit U, Demir K, Karaman E, Hamzaoglu AI, Bakirer G. Developmental competence of domestic cat oocytes from ovaries stored at various durations at 4 degrees C temperature. Anim Reprod Sci. 2009;116:169–72.
Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41.
Menze MA, Chakraborty N, Clavenna M, Banerjee M, Liu XH, Toner M, et al. Metabolic preconditioning of cells with AICAR-riboside: improved cryopreservation and cell-type specific impacts on energetics and proliferation. Cryobiology. 2010;61:79–88.
Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–21.
Weisová P, Anilkumar U, Ryan C, Concannon CG, Prehn JHM, Ward MW. ‘Mild mitochondrial uncoupling’ induced protection against neuronal excitotoxicity requires AMPK activity. Biochim Biophys Acta. 1817;2012:744–53.
Tanpradit N, Comizzoli P, Srisuwatanasagul S, Chatdarong K. Positive impact of sucrose supplementation during slow freezing of cat ovarian tissues on cellular viability, follicle morphology, and DNA integrity. Theriogenology. 2015;83:1553–61.
Fujihara M, Comizzoli P, Keefer CL, Wildt DE, Songsasen N. Epidermal growth factor (EGF) sustains in vitro primordial follicle viability by enhancing stromal cell proliferation via MAPK and PI3K pathways in the prepubertal, but not adult, cat ovary. Biol Reprod. 2014;90:86.
Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrere S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril. 2004;82:1390–4.
Han YH, Kim SH, Kim SZ, Park WH. Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) as an O2(*-) generator induces apoptosis via the depletion of intracellular GSH contents in Calu-6 cells. Lung Cancer. 2009;63:201–9.
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115.
Cottet-Rousselle C, Ronot X, Leverve X, Mayol JF. Cytometric assessment of mitochondria using fluorescent probes. Cytometry A. 2011;79:405–25.
Gan Z, Audi SH, Bongard RD, Gauthier KM, Merker MP. Quantifying mitochondrial and plasma membrane potentials in intact pulmonary arterial endothelial cells based on extracellular disposition of rhodamine dyes. Am J Physiol Lung Cell Mol Physiol. 2011;300:L762–72.
Kenngott RA, Vermehren M, Ebach K, Sinowatz F. The role of ovarian surface epithelium in folliculogenesis during fetal development of the bovine ovary: a histological and immunohistochemical study. Sex Dev. 2013;7:180–95.
Quarrie R, Lee DS, Reyes L, Erdahl W, Pfeiffer DR, Zweier JL, et al. Mitochondrial uncoupling does not decrease reactive oxygen species production after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2014;307:H996–1004.
Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab. 2014;3:114–23.
Otala M, Erkkila K, Tuuri T, Sjoberg J, Suomalainen L, Suikkari AM, et al. Cell death and its suppression in human ovarian tissue culture. Mol Hum Reprod. 2002;8:228–36.
Fabbri R, Sapone A, Paolini M, Vivarelli F, Franchi P, Lucarini M, et al. Effects of N-acetylcysteine on human ovarian tissue preservation undergoing cryopreservation procedure. Histol Histopathol. 2015;30:725–35.
da Silva Caldeira CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–60.
Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29:1931–40.
Meirow D, Roness H, Kristensen SG, Andersen CY. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum Reprod. 2015;30:2453–6.
Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction. 2011;141:183–91.
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–9.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15.
Brennan JP, Berry RG, Baghai M, Duchen MR, Shattock MJ. FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarisation. Cardiovasc Res. 2006;72:322–30.
Fujihara M, Comizzoli P, Wildt DE, Songsasen N. Cat and dog primordial follicles enclosed in ovarian cortex sustain viability after in vitro culture on agarose gel in a protein-free medium. Reprod Domest Anim. 2012;47 Suppl 6:102–8.
Mlejnek P, Dolezel P. Loss of mitochondrial transmembrane potential and glutathione depletion are not sufficient to account for induction of apoptosis by carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone in human leukemia K562 cells. Chem Biol Interact. 2015;239:100–10.
Han YH, Park WH. Intracellular glutathione levels are involved in carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone-induced apoptosis in As4.1 juxtaglomerular cells. Int J Mol Med. 2011;27:575–81.
Henry L, Fransolet M, Labied S, Blacher S, Masereel MC, Foidart JM, et al. Supplementation of transport and freezing media with anti-apoptotic drugs improves ovarian cortex survival. J Ovarian Res. 2016;9:4.
Sanfilippo S, Canis M, Romero S, Sion B, Dechelotte P, Pouly JL, et al. Quality and functionality of human ovarian tissue after cryopreservation using an original slow freezing procedure. J Assist Reprod Genet. 2013;30:25–34.
Khosravi F, Reid RL, Moini A, Abolhassani F, Valojerdi MR, Kan FWK. In vitro development of human primordial follicles to preantral stage after vitrification. J Assist Reprod Genet. 2013;30:1397–406.
Maro B, Marty MC, Bornens M. In vivo and in vitro effects of the mitochondrial uncoupler FCCP on microtubules. EMBO J. 1982;1:1347–52.
Alieva IB, Vorobjev IA. Centrosome behavior under the action of a mitochondrial uncoupler and the effect of disruption of cytoskeleton elements on the uncoupler-induced alterations. J Struct Biol. 1994;113:217–24.
Yeste M, Estrada E, Rocha LG, Marin H, Rodriguez-Gil JE, Miro J. Cryotolerance of stallion spermatozoa is related to ROS production and mitochondrial membrane potential rather than to the integrity of sperm nucleus. Andrology. 2015;3:395–407.
Jones A, Van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod. 2004;19:1861–6.
Lei T, Guo N, Tan MH, Li YF. Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J Huazhong Univ Sci Technol Med Sci. 2014;34:99–102.
Kim SS, Kang HG, Kim NH, Lee HC, Lee HH. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20:2502–8.
Isachenko V, Isachenko E, Mallmann P, Rahimi G. Increasing follicular and stromal cell proliferation in cryopreserved human ovarian tissue after long-term precooling prior to freezing: in vitro versus chorioallantoic membrane (CAM) xenotransplantation. Cell Transplant. 2013;22:2053–61.
Guimaraes EL, Best J, Dolle L, Najimi M, Sokal E, van Grunsven LA. Mitochondrial uncouplers inhibit hepatic stellate cell activation. BMC Gastroenterol. 2012;12:68.
Milenkovic M, Diaz-Garcia C, Wallin A, Brannstrom M. Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertil Steril. 2012;97:1176–82.
Luyckx V, Scalercio S, Jadoul P, Amorim CA, Soares M, Donnez J, et al. Evaluation of cryopreserved ovarian tissue from prepubertal patients after long-term xenografting and exogenous stimulation. Fertil Steril. 2013;100:1350–7.
Jewgenow K, Göritz F. The recovery of preantral follicles from ovaries of domestic cats and their characterisation before and after culture. Anim Reprod Sci. 1995;39:285–97.
Aguiar FL, Lunardi FO, Lima LF, Rocha RM, Bruno JB, Magalhaes-Padilha DM, et al. Insulin improves in vitro survival of equine preantral follicles enclosed in ovarian tissue and reduces reactive oxygen species production after culture. Theriogenology. 2016;85:1063–9.
Kim EJ, Lee HJ, Lee J, Youm HW, Lee JR, Suh CS, et al. The beneficial effects of polyethylene glycol-superoxide dismutase on ovarian tissue culture and transplantation. J Assist Reprod Genet. 2015;32:1561–9.
Acknowledgements
The authors would like to thank Asst. Prof. Dr. Sayamon Srisuwatanasagul for histologic technical assistance, Dr. Paweena Thuwanut for histologic interpretation advice and Assoc. Prof. Dr. Padet Tummaruk for helping with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support for this work was provided by the Royal Golden Jubilee Ph.D. Program (PHD/0199/2552), the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), and Research Unit of Obstetrics and Reproduction in Animals, Chulalongkorn University.
Additional information
Capsule Uncoupling the oxidative phosphorylation of the domestic cat ovarian tissue with carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) is beneficial to the viability and morphology of follicles during ovarian tissue culture. However, tissue pre-exposure to FCCP does not have a protective or detrimental effect during cryopreservation.
Rights and permissions
About this article
Cite this article
Tanpradit, N., Chatdarong, K. & Comizzoli, P. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) pre-exposure ensures follicle integrity during in vitro culture of ovarian tissue but not during cryopreservation in the domestic cat model. J Assist Reprod Genet 33, 1621–1631 (2016). https://doi.org/10.1007/s10815-016-0810-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0810-5