Abstract
Purpose
Using a rabbit model, we assessed the influence of sperm DNA longevity on female reproductive outcomes.
Methods
Semen was collected from 40 bucks, incubated at 38 °C for 24 h, and the rate of sperm DNA fragmentation (rSDF) was determined using the sperm chromatin dispersion assay. Males were allocated into high rSDF (>0.5 units of increase per hour) or low rSDF (<0.5 units of increase per hour) groups. High and low rSDF semen samples were sequentially artificially inseminated into the same doe to reduce female factor variability, and pregnancy outcomes were recorded.
Results
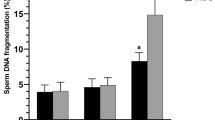
While there was no difference in SDFs between rSDF groups immediately after collection (T0), differences were significant after 2 h of incubation; SDFs determined at collection and rSDF behaved as independent characters (Pearson correlation = 0.099; P = 0.542). Following artificial insemination, the rate of stillborn pups was significantly higher in does inseminated by males with a high rSDF (14/21) compared to those with low rSDF (15/6); (contingency χ2 5.19; p = 0.022). The risk of stillborn when low rSDF rabbits were used for insemination was 0.16, but increased to 0.36 when high rSDF animals were used (odds ratio = 2.85; 95 % confidence interval = 1.4–2.7).
Conclusion(s)
Dynamic assessment of SDF coupled with natural multiple ovulation, high fecundity of the rabbit and control over female factor influence, provided a useful experimental model to demonstrate the adverse effect of reduced sperm DNA longevity on reproductive outcome.


Similar content being viewed by others
References
Drobnis EZ, Johnson MH. Are we ready to incorporate sperm DNA-fragmentation testing into our male infertility work-up? A plea for more robust studies. Reprod Biomed Online. 2015;30:111–2.
Palermo GD, Neri QV, Cozzubbo T, Rosenwaks Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil Steril. 2014;102:1508–17.
Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17.
Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85.
Santiso R, Tamayo M, Gosálvez J, Johnston S, Mariño A, Fernández C, et al. DNA fragmentation dynamics allows the assessment of cryptic sperm damage in human: evaluation of exposure to ionizing radiation, hyperthermia, acidic pH and nitric oxide. Mutat Res. 2012;734:41–9.
Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7.
Oliva R. Protamines and male infertility. Hum Reprod. 2006;12:417–35.
Gosálvez J, López-Fernández C, Fernández JL, Gouraud A, Holt WV. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian sperm from eleven species. Mol Reprod Dev. 2011;78:951–61.
Gosálvez J, Holt WV, Johnston SD. Sperm DNA fragmentation and its role in wildlife conservation. Reproductive sciences in animal conservation. Adv Exp Med Biol. 2014;753:357–84.
Gosálvez J, López-Fernández C, Arroyo F, Gosálbez A, Gutiérrez-Cortés El, Johnston SD. The assessment of sperm DNA damage in rabbits using the Halomax assay. In: Adamo G, Constanza A, editors. Rabbits: biology, diet and eating habits and disorders. New York: NOVA Science publishers; 2013. p. 87–100.
Bencheik N. Effect de la fréquence de collecte de la semence sur les caractéristiques du sperme et des spermatozoides récoltés chez la lapin. Ann Zootech. 1995;44:263–79.
Mocé E, Vicente JS, Lavara R. Effect of donor strain and maturation stage of rabbit oocytes on results of penetration test of rabbit semen. World Rabbit Sci. 2002;10:53–62.
Gosálvez J, López-Fernández C, Fernández JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotech Fertil. 2015;4:1–16.
Aurich C. Recent advances in cooled-semen technology. Anim Reprod Sci. 2008;107:268–75.
López-Fernández C, Gage MJ, Arroyo F, Gosálbez A, Larrán AM, Fernández JL, et al. Rapid rates of sperm DNA damage after activation in tench (Tinca tinca: Teleostei, Cyprinidae) measured using a sperm chromatin dispersion test. Reproduction. 2009;138:257–66.
Wdowiak A, Bojar I. Relationship between pregnancy, embryo development, and sperm deoxyribonucleic acid fragmentation dynamics. Saudi J Biol Sci. 2015 (in press). Published online ahead of print 10 August 2015. doi:10.1016/j.sjbs.2015.08.001.
Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005.
Leach M, Aitken RJ, Sacks G. Sperm DNA fragmentation abnormalities in men from couples with a history of recurrent miscarriage. Aust NZ J Obst Gyn. 2015;55:379–83.
Gosálvez J, Caballero P, López-Fernández C, Ortega L, Guijarro JA, Fernández JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15:812–8.
Genescà A, Caballín MR, Miró R, Benet J, Germà JR, Egozcue J. Repair of human sperm chromosome aberrations in the hamster egg. Hum Genet. 1992;89:181–6.
Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–37.
Marchetti F, Bishop J, Gingerich J, Wyrobek AJ. Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair. Sci Rep. 2015;5:7689.
Gosálvez J, López-Fernández C, Hermoso A, Fernández JL, Kjelland ME. Sperm DNA fragmentation in zebrafish (Danio rerio) and its impact on fertility and embryo viability —Implications for fisheries and aquaculture. Aquaculture. 2014;433:173–82.
Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction. 2010;139:989–97.
Devaux A, Fiat L, Gillet C, Bony S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and arctic charr (Salvelinus alpinus). Aquat Toxicol. 2011;101:405–11.
Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramırez MA, Pericuesta E, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–72.
De Rycke M, Liebaers I, Van Steirteghem A. Epigenetic risks related to assisted reproductive technologies. Risk analysis and epigenetic inheritance. Hum Reprod. 2002;17:2487–94.
Kelly TL, Trasler JM. Reproductive epigenetics. Clin Genet. 2004;65:247–60.
Urrego R, Rodriguez-Osorio N, Niemann H. Epigenetic disorders and altered gene expression after use of assisted reproductive technologies in domestic cattle. Epigenetics. 2014;9:803–15.
Acknowledgments
This research was supported by the Spanish Ministry of Economy and Competitiveness, MINECO (BFU-2013-44290-R).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Sequential artificial insemination of the same naturally multi-ovulating female makes the rabbit a useful model for investigating the effect of sperm DNA longevity on reproductive outcome. Decreased sperm DNA longevity resulted in a diminished proportion of live offspring per pregnancy and an increased number of stillborn kittens.
Rights and permissions
About this article
Cite this article
Johnston, S.D., López-Fernández, C., Arroyo, F. et al. Reduced sperm DNA longevity is associated with an increased incidence of still born; evidence from a multi-ovulating sequential artificial insemination animal model. J Assist Reprod Genet 33, 1231–1238 (2016). https://doi.org/10.1007/s10815-016-0754-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0754-9