Abstract
Purpose
The study aims to assess the protective effects of dimethyl sulfoxide (DMSO)-free solution based on trehalose on the cryopreservation of a whole sheep ovary and evaluate its use as an efficient cryoprotectant.
Method
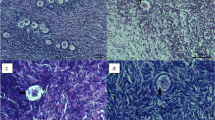
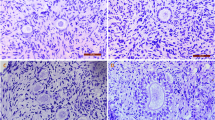
Twenty-one ovaries collected from 6- to 8-month-old non-pregnant female sheep were randomly distributed into three groups, namely, a fresh group, a DMSO-free group, and a DMSO group. The morphology, cell apoptosis (by hematoxylin and eosin (HE) staining and terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) assay), and mRNA transcript of Bcl-2-associated X protein (BAX) and cold inducible RNA-binding protein (CIRP) (by real-time PCR) of the thawed sheep ovaries and fresh controls were tested to establish a criterion for appraising the results of the cryopreservation.
Results
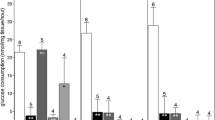
(i) The histological assessment indicated that the structure of the DMSO-free ovaries remained largely intact and comparable to those of the fresh control groups; whereas, significant damage was observed in the ovaries of the DMSO group (P < 0.05). (ii) The TUNEL assay and mRNA transcript of the BAX assessment showed that the apoptosis parameter in the fresh group was the lowest among all the groups (P < 0.05), and the parameter in the DMSO-free group was significantly lower than that in the DMSO group (P < 0.05). (iii) The level of the CIRP transcripts increased the most in the DMSO-free group followed by the DMSO group and the fresh control group (P < 0.05).
Conclusions
These results indicate that a DMSO-free cryoprotectant solution, especially a trehalose cryoprotectant, is an efficient cryoprotectant and has a beneficial effect on the cryopreservation of whole sheep ovaries.






Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–44.
Macklon KT, Jensen AK, Loft A, et al. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–64.
Ernst E, Bergholdt S, Jorgensen JS, et al. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25(5):1280–1.
Van Eyck AS, Bouzin C, Feron O, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–85.
Van Eyck AS, Jordan BF, Gallez B, et al. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–81.
Imhof M, Bergmeister H, Lipovac M, et al. Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertil Steril. 2006;85 Suppl 1:1208–15.
Campbell BK, Hernandez-Medrano J, Onions V, et al. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 2014;29(8):1749–63.
Windrum P, Morris TC. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31(4):315.
Hakura A, Mochida H, Yamatsu K. Dimethyl sulfoxide (DMSO) is mutagenic for bacterial mutagenicity tester strains. Mutat Res. 1993;303(3):127–33.
Schiraldi C, Di Lernia I, De Rosa M. Trehalose production: exploiting novel approaches. Trends Biotechnol. 2002;20(10):420–5.
Eroglu A, Russo MJ, Bieganski R, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol. 2000;18(2):163–7.
Eroglu A, Bailey SE, Toner M, et al. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol Reprod. 2009;80(1):70–8.
Satpathy GR, Torok Z, Bali R, et al. Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology. 2004;49(2):123–36.
Hu JH, Zan LS, Zhao XL, et al. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J Anim Sci. 2010;88(5):1657–62.
Lu H, Zhu Z, Dong L, et al. Lack of trehalose accelerates H2O2-induced Candida albicans apoptosis through regulating Ca2+ signaling pathway and caspase activity. PLoS ONE. 2011;6(1):e15808.
Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem. 2001;276(26):24261–7.
Torre A, Momier M, Mazoyer C, et al. Validation of a new metabolic marker to assess the vascular viability of vitrified whole sheep ovaries. Hum Reprod. 2012;27(6):1811–21.
Revel A, Elami A, Bor A, et al. Whole sheep ovary cryopreservation and transplantation. Fertil Steril. 2004;82(6):1714–5.
Zhang JM, Zhang YC, Ruan LH, et al. Optimizing cryoprotectant perfusion conditions for intact ovary: a bovine model. J Assist Reprod Genet. 2012;29(11):1255–60.
Bedaiwy MA, Jeremias E, Gurunluoglu R, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003;79(3):594–602.
Courbiere B, Massardier J, Salle B, et al. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84 Suppl 2:1065–71.
Zhang JM, Sheng Y, Cao YZ, et al. Cryopreservation of whole ovaries with vascular pedicles: vitrification or conventional freezing? J Assist Reprod Genet. 2011;28(5):445–52.
Xu Z, Wang X, Wu Y, et al. Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries. Theriogenology. 2012;77(3):483–91.
Martino M, Morabito F, Messina G, et al. Fractionated infusions of cryopreserved stem cells may prevent DMSO-induced major cardiac complications in graft recipients. Haematologica. 1996;81(1):59–61.
Wang HY, Lun ZR, Lu SS. Cryopreservation of umbilical cord blood-derived mesenchymal stem cells without dimethyl sulfoxide. Cryo Lett. 2011;32(1):81–8.
Jain NK, Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18(1):24–36.
Branca C, Maccarrone S, Magazu S, et al. Tetrahedral order in homologous disaccharide-water mixtures. J Chem Phys. 2005;122:17451317.
Zhao J, Wang S, Bao J, et al. Trehalose maintains bioactivity and promotes sustained release of BMP-2 from lyophilized CDHA scaffolds for enhanced osteogenesis in vitro and in vivo. PLoS ONE. 2013;8(1):e54645.
Nguyen TM, Kikuchi K, Nakai M, et al. Effect of trehalose on DNA integrity of freeze-dried boar sperm, fertilization, and embryo development after intracytoplasmic sperm injection. Theriogenology. 2013;80(9):1033–44.
Matsuo K, Takahashi T, Igarashi H, et al. Effects of different trehalose concentrations in a warming medium on embryo survival and clinical outcomes in vitrified human embryos. Gynecol Obstet Investig. 2013;76(4):214–20.
Amirat L, Tainturier D, Jeanneau L, et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl, a commercial egg yolk extender. Theriogenology. 2004;61(5):895–907.
Moussa M, Marinet V, Trimeche A, et al. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology. 2002;57(6):1695–706.
Graham JK, Foote RH. Effect of several lipids, fatty acyl chain length, and degree of unsaturation on the motility of bull spermatozoa after cold shock and freezing. Cryobiology. 1987;24(1):42–52.
Johnson LA, Weitze KF, Fiser P, et al. Storage of boar semen. Anim Reprod Sci. 2000;62(1–3):143–72.
Dasiman R, Rahman NS, Othman S, et al. Cytoskeletal alterations in different developmental stages of in vivo cryopreserved preimplantation murine embryos. Med Sci Monit Basic Res. 2013;19:258–66.
de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci USA. 2009;106(10):3654–8.
Lee YA, Kim YH, Kim BJ, et al. Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS ONE. 2013;8(1):e54889.
Liu Q, Xu L, Jiao S, et al. Trehalose inhibited the phagocytosis of refrigerated platelets in vitro via preventing apoptosis. Transfusion. 2009;49(10):2158–66.
Al-Fageeh MB, Smales CM. Cold-inducible RNA binding protein (CIRP) expression is modulated by alternative mRNAs. RNA. 2009;15(6):1164–76.
Li S, Zhang Z, Xue J, et al. Cold-inducible RNA binding protein inhibits H2O2-induced apoptosis in rat cortical neurons. Brain Res. 2012;1441:47–52.
Sakurai T, Itoh K, Higashitsuji H, et al. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta. 2006;1763(3):290–5.
Acknowledgments
The work of this article was done in the Frozen Laboratory of Qilu Hospital of Shandong University.
The authors are grateful to the Shandong Province Science and Technology Research Project (2010GSF10814) and to the National Science Council for financially supporting this study (81370711); the Frozen Laboratory of Qilu Hospital of Shandong University generously provided relevant experimental technology for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
These results indicate that a DMSO-free and trehalose-containing cryoprotectant solution has a beneficial effect on the cryopreservation of intact sheep ovaries.
Tianqi Du and Lan Chao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Du, T., Chao, L., Zhao, S. et al. Successful cryopreservation of whole sheep ovary by using DMSO-free cryoprotectant. J Assist Reprod Genet 32, 1267–1275 (2015). https://doi.org/10.1007/s10815-015-0513-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-015-0513-3