Abstract
Purpose
Fertility preservation strategies warrant non-invasive viability assessment of preantral follicles (PAF) such as staining with Neutral Red (NR) that is incorporated by viable follicles. To optimize the procedure, we firstly determined the lowest concentration and shortest exposure time needed for optimal viability screening of isolated bovine PAF. Secondly, we combined this protocol to a vitrification procedure to assess cryotolerance of the stained follicles.
Methods
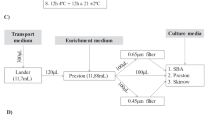
Isolated PAF (900, divided over 6 replicates) were cultured in DMEM/Ham’s F12 (Culture Medium - Cm) for 4 days (38.5 °C, 5 % CO2). On D0, D2 and D4, follicles were stained, by adding NR medium (NRm = Cm with different concentrations NR) after which viability was assessed by counting stained/non-stained PAF every 30 min for a period of 2 h.
Results
Following a binary logistic regression analysis with staining as a result (yes/no) versus log-concentration, a probability model could be fitted, indicating that the proportion of stained follicles remained stable after 30 min when 15 μg/ml NR was used, without compromising follicular health and viability. Consequently, using this protocol, no significant effect of staining prior to vitrification, was found on PAF viability immediately after warming or following 4 days of culture.
Conclusions
In conclusion, we propose NR staining as a non-invasive, non-detrimental viability assessment tool for PAF, when applied at 15 μg/ml for 30 min, being perfectly compatible with PAF vitrification.








Similar content being viewed by others
References
Fortune JE, Kito S, Wandji SA, Srsen V. Activation of bovine and baboon primordial follicles in vitro. Theriogenology. 1998;49(2):441–9.
Campos JR, Rosa ESAC. Cryopreservation and fertility: current and prospective possibilities for female cancer patients. ISRN Obstet Gynecol. 2011;2011:350813. doi:10.5402/2011/350813.
Vanacker J, Luyckx V, Amorim C, Dolmans MM, Van Langendonckt A, Donnez J, et al. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril. 2013;99(5):1363–8 e2. doi:10.1016/j.fertnstert.2012.12.016.
Amorim CA, Goncalves PB, Figueiredo JR. Cryopreservation of oocytes from pre-antral follicles. Hum Reprod Update. 2003;9(2):119–29.
Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, et al. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl. 2003;61:429–43.
Malhi PS, Adams GP, Singh J. Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol Reprod. 2005;73(1):45–53. doi:10.1095/biolreprod.104.038745.
Aerts JM, De Clercq JB, Andries S, Leroy JL, Van Aelst S, Bols PE. Follicle survival and growth to antral stages in short-term murine ovarian cortical transplants after Cryologic solid surface vitrification or slow-rate freezing. Cryobiology. 2008;57(2):163–9. doi:10.1016/j.cryobiol.2008.07.011.
Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Human reproduction (Oxford, England). 1999;14(10):2519–24.
Park KS, Lee TH, Park YK, Song HB, Chun SS. Effects of isolating methods (mechanical or enzymatical) on structure of pre-antral follicles in mouse. J Assist Reprod Genet. 2005;22(9–10):355–9. doi:10.1007/s10815-005-6796-z.
Chambers EL, Gosden RG, Yap C, Picton HM. In situ identification of follicles in ovarian cortex as a tool for quantifying follicle density, viability and developmental potential in strategies to preserve female fertility. Hum Reprod (Oxford, England). 2010;25(10):2559–68. doi:10.1093/humrep/deq192.
Sanfilippo S, Canis M, Ouchchane L, Botchorishvili R, Artonne C, Janny L, et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1). J Assist Reprod Genet. 2011;28(12):1151–6. doi:10.1007/s10815-011-9649-y.
Fransolet M, Labied S, Henry L, Masereel MC, Rozet E, Kirschvink N, et al. Strategies for using the sheep ovarian cortex as a model in reproductive medicine. PloS one. 2014;9(3):e91073. doi:10.1371/journal.pone.0091073.
Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod (Oxford, England). 2011;26(1):157–66. doi:10.1093/humrep/deq318.
Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. 2008;3(7):1125–31. doi:10.1038/nprot.2008.75.
Elliott WM, Auersperg N. Comparison of the neutral red and methylene blue assays to study cell growth in culture. Biotechnic Histochem : Off Publ Biol Stain Comm. 1993;68(1):29–35. doi:10.3109/10520299309105573.
Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24(2–3):119–24.
Santos RR, Amorim C, Cecconi S, Fassbender M, Imhof M, Lornage J, et al. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci. 2010;122(3–4):151–63. doi:10.1016/j.anireprosci.2010.08.010.
Torre A, Momier M, Mazoyer C, Selva J, Salle B, Lornage J. Validation of a new metabolic marker to assess the vascular viability of vitrified whole sheep ovaries. Hum Reprod (Oxford, England). 2012;27(6):1811–21. doi:10.1093/humrep/des100.
Xu Z, Wang X, Wu Y, Meng Y, Wu F, Zhou N, et al. Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries. Theriogenology. 2012;77(3):483–91. doi:10.1016/j.theriogenology.2011.08.017.
Bromer JG, Patrizio P. Fertility preservation: the rationale for cryopreservation of the whole ovary. Semin Reprod Med. 2009;27(6):465–71. doi:10.1055/s-0029-1241056.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi:10.1016/j.fertnstert.2013.03.030.
Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod (Oxford, England). 2013;28(5):1267–79. doi:10.1093/humrep/det032.
Langbeen A, Jorssen EPA, Fransen E, Rodriguez APA, Chong Garcìa M, Leroy JLMR et al. Characterization of freshly retrieved preantral follicles using a low-invasive, mechanical isolation method extended to different ruminant species. Zygote. 2014.
E.P.A. Jorssen AL, J.L.M.R. Leroy, S. Andries, E. Merckx, P.E.J. Bols, editor. The effect of FSH on the survival and growth of individually in vitro cultured early pre-antral bovine follicles. REPRODUCTION IN DOMESTIC ANIMALS 2013 september 2013; Bologna.
Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE. Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology. 2014. doi:10.1016/j.theriogenology.2014.04.019.
Studies RRB. on the pancreas of the Guinea Pig. American. J Anat. 1911;12:297.
Gray DW, Millard PR, McShane P, Morris PJ. The use of the dye neutral red as a specific, non-toxic, intra-vital stain of islets of Langerhans. Br J Exp Pathol. 1983;64(5):553–8.
Lee Jr WH, Hagerty RF, Braid HL. Measurements of cellular viability. A comparative study of neutral red and radioactive sulfate in the examination of the viability of cartilage. Plast Reconstr Surg Transplant Bull. 1960;26:280–5.
Maier K, Schmitt-Landgraf R, Siegemund B. Development of an in vitro test system with human skin cells for evaluation of phototoxicity. Toxicol In Vitro : Int J Published Assoc BIBRA. 1991;5(5–6):457–61.
Fautz R, Husein B, Efstathiou E, Hechenberger-Freudl C. Assessment of the relation between the initial viability and the attachment of freshly isolated rat hepatocytes used for the in vivo/in vitro DNA repair assay (UDS). Mutat Res. 1993;291(1):21–7.
Zhang SZ, Lipsky MM, Trump BF, Hsu IC. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes. Cell Biol Toxicol. 1990;6(2):219–34.
Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160(2):171–7. doi:10.1016/j.toxlet.2005.07.001.
Rodrigues RM, Bouhifd M, Bories G, Sacco MG, Gribaldo L, Fabbri M, et al. Assessment of an automated in vitro basal cytotoxicity test system based on metabolically-competent cells. Toxicol In Vitro : Int J Published Assoc BIBRA. 2013;27(2):760–7. doi:10.1016/j.tiv.2012.12.004.
Adams GP, Pierson RA. Bovine model for study of ovarian follicular dynamics in humans. Theriogenology. 1995;43(1):113–20. doi:10.1016/0093-691X(94)00015-M.
Menezo YJ, Herubel F. Mouse and bovine models for human IVF. Reprod Biomed Online. 2002;4(2):170–5.
Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62(5):1322–8.
McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reprod (Cambridge, England). 2010;139(6):971–8. doi:10.1530/REP-10-0025.
Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59(4):783–90.
Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod (Oxford, England). 2008;23(5):1151–8. doi:10.1093/humrep/den070.
Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod (Oxford, England). 2011;26(5):1061–72. doi:10.1093/humrep/der049.
Albertini DF, Akkoyunlu G. Ovarian follicle culture systems for mammals. Methods Enzymol. 2010;476:107–21. doi:10.1016/S0076-6879(10)76007-9.
Hartshorne GM. In vitro culture of ovarian follicles. Rev Reprod. 1997;2(2):94–104.
Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod (Oxford, England). 1996;11(12):2656–66.
Guerard M, Zeller A, Singer T, Gocke E. In vitro genotoxicity of neutral red after photo-activation and metabolic activation in the Ames test, the micronucleus test and the comet assay. Mutat Res. 2012;746(1):15–20. doi:10.1016/j.mrgentox.2012.01.012.
Soleimani R, De Vos W, Van Oostveldt P, Lierman S, Van den Broecke R, De Sutter P, et al. Two novel techniques to detect follicles in human ovarian cortical tissue. Hum Reprod (Oxford, England). 2006;21(7):1720–4. doi:10.1093/humrep/del057.
Norins AL, Gould WM. The photodynamic action of neutral red on rabbit basophils. J Investig Dermatol. 1964;42:257–9.
Varghese AC, du Plessis SS, Falcone T, Agarwal A. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol : RB&E. 2008;6:47. doi:10.1186/1477-7827-6-47.
Arav A. Cryopreservation of oocytes and embryos. Theriogenology. 2014;81(1):96–102. doi:10.1016/j.theriogenology.2013.09.011.
Celestino JJ, dos Santos RR, Lopes CA, Martins FS, Matos MH, Melo MA, et al. Preservation of bovine preantral follicle viability and ultra-structure after cooling and freezing of ovarian tissue. Anim Reprod Sci. 2008;108(3–4):309–18. doi:10.1016/j.anireprosci.2007.08.016.
Liebenthron J, Koster M, Drengner C, Reinsberg J, van der Ven H, Montag M. The impact of culture conditions on early follicle recruitment and growth from human ovarian cortex biopsies in vitro. Fertility and sterility. 2013:483–91. doi:10.1016/j.fertnstert.2013.03.046.
Oskam IC, Lund T, Santos RR. Irreversible damage in ovine ovarian tissue after cryopreservation in propanediol: analyses after in vitro culture and xenotransplantation. Reprod Domest Anim. 2011;46(5):793–9. doi:10.1111/j.1439-0531.2010.01743.x.
Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Preservation of human ovarian follicles within tissue frozen by vitrification in a xeno-free closed system using only ethylene glycol as a permeating cryoprotectant. Fertility and sterility. 2013:170–7. doi:10.1016/j.fertnstert.2013.03.018.
Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103(2):378–86. doi:10.1002/bit.22250.
Amorim CA, Rondina D, Rodrigues AP, Costa SH, Goncalves PB, de Figueiredo JR, et al. Isolated ovine primordial follicles cryopreserved in different concentrations of ethylene glycol. Theriogenology. 2003;60(4):735–42.
Amorim CA, Rodrigues AP, Rondina D, Goncalves PB, de Figueiredo JR, Giorgetti A. Cryopreservation of ovine primordial follicles using dimethyl sulfoxide. Fertil Steril. 2003;79 Suppl 1:682–6.
Lamaita RM, Bambirra EA, Camargos M, Silva-Filho AL, Reis FM, Camargos AF. Histological evaluation of the effects of cryopreservation in bovine ovarian tissue. J Assist Reprod Genet. 2005;22(2):105–6.
Paynter SJ, Cooper A, Fuller BJ, Shaw RW. Cryopreservation of bovine ovarian tissue: structural normality of follicles after thawing and culture in vitro. Cryobiology. 1999;38(4):301–9. doi:10.1006/cryo.1999.2170.
Abedelahi A, Salehnia M, Allameh AA, Davoodi D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod (Oxford, England). 2010;25(4):977–85. doi:10.1093/humrep/deq002.
Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol. 2006;21(8):887–98.
Donnez J, Dolmans MM. Cryopreservation of ovarian tissue: an overview. Minerva Med. 2009;100(5):401–13.
Oktem O, Oktay K. Fertility preservation for breast cancer patients. Semin Reprod Med. 2009;27(6):486–92. doi:10.1055/s-0029-1241059.
Acknowledgments
The authors thank Silke Andries and Els Merckx for their excellent technical assistance and the local slaughterhouses for their cooperation in sample collection.
Conflict of interest
All (co-)authors state that the funding of this research is provided by the independent Operational Costs of the University of Antwerp. E. P. A. Jorssen acknowledges support from a Research Grant from the Belgian Government (Federale Overheidsdienst Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu, Cel Contractueel Onderzoek) ‘Embryoscreen RF6222’. There is nothing to disclose and there are no conflicts of interest, financially nor personal, for none of the (co-)authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Applied at a concentration of 15 μg/ml for 30 minutes, Neutral Red is proposed as a non-invasive viability assessment tool for isolated bovine preantral follicles, without compromising follicle cryotolerance.
Rights and permissions
About this article
Cite this article
Langbeen, A., Jorssen, E., Granata, N. et al. Effects of neutral red assisted viability assessment on the cryotolerance of isolated bovine preantral follicles. J Assist Reprod Genet 31, 1727–1736 (2014). https://doi.org/10.1007/s10815-014-0340-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0340-y