Abstract
Main purpose and research question
To determine whether the true fusogen Syncytin-1 and its receptor (ASCT-2) is present in human gametes using qRT-PCR, immunoblotting and immunofluorescence.
Methods
Donated oocytes and spermatozoa, originating from a fertility center in tertiary referral university hospital, underwent qRT-PCR, immunoblotting and immunofluorescence analyzes.
Results
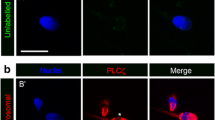
Quantitative RT-PCR of sperm samples from sperm donors showed that syncytin-1 is present in all samples, however, protein levels varied between donors. Syncytin-1 immunoreactivity predominates in the sperm head and around the equatorial segment. The receptor ASCT-2 is expressed in the acrosomal region and in the sperm tail. Moreover, ASCT-2, but not syncytin-1, is expressed in oocytes and the mRNA level increases with increasing maturity of the oocytes.
Conclusions
Syncytin and its receptor are present in human gametes and localization and temporal appearance is consistent with a possible role in fusion between oocyte and sperm.




Similar content being viewed by others
References
Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The physiology of reproduction. 2nd ed. New York: Raven; 1994. p. 189–317.
van Gestel RA, Brewis IA, Ashton PR, Brouwers JF, Gadella BM. Multiple proteins present in purified porcine sperm apical plasmamembranes interact with the zona pellucida of the oocyte. Mol Hum Reprod. 2007;13:445–54.
Nixon B, Mitchell LA, Anderson AL, Mclaughlin EA, O’bryan MK, Aitken RJ. Proteomic and functional analysis of human sperm detergent resistant membranes. J Cell Physiol. 2011;226:2651–65.
Blas GAD, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biol. 2005;3:e323.
Rodriguez F, Bustos MA, Zanetti MN, Ruete MC, Mayorga LS, Tomes CN. alfa-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. PLoS One. 2011;6:e21925.
Nixon B, Aitken RJ, McLaughlin EA. New insights into the molecular mechanisms of sperm-egg interaction. Cell Mol Life Sci. 2007;64:1805–23.
Sutovsky P. Sperm–egg adhesion and fusion in mammals. Expert Rev Mol Med. 2009;11:e11.
Evans JP. Sperm-egg interaction. Annu Rev Physiol. 2012;74:477–502.
Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–8.
Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, et al. Reduced fertility of female mice lacking CD81. Dev Biol. 2006;290:351–8.
Yunta M, Lazo PA. Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell Signal. 2003;15:559–64.
Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–46.
Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–5.
Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–9.
Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–9.
Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, et al. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A. 2009;106:12127–32.
Bjerregaard B, Talts JF, Larsson LI. The endogenous envelope protein syncytin is involved in myoblast fusion. In: Larsson LI, editor. Cell fusions, regulation and control. New York: Springer; 2011. p. 267–75.
Søe K, Andersen TL, Hobolt-Pedersen AS, Bjerregaard B, Larsson LI, Delaissé JM. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone. 2011;48:837–46.
Bjerregaard B, Holck S, Christensen IJ, Larsson LI. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63:1906–11.
Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral syncytin-1 and regulated by TGF-beta. J Mol Med. 2007;85:23–38.
Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol. 2000;76:6442–52.
Mortensen K, Lichtenberg J, Thomasen PD, Larsen LI. Spontaneous fusion between cancer cells and endothelial cells. Cell Mol Life Sci. 2004;61:2125–31.
Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277:50062–8.
Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–94.
Mallet PJ, Stock CE, Fraser LR. Acrosome loss in human sperm incubated in vitro under capacitating conditions. Int J Androl. 1985;8:357–64.
Green S, Fishel S, Rowe P. The incidence of spontaneous acrosome reaction in homogeneous populations of hyperactivated human spermatozoa. Hum Reprod. 1999;14:1819–22.
Bedford JM, Cooper GW. Membrane fusion events in the fertilization of vertebrate eggs. In: Poste G, Nicholson GL, editors. Cell surface reviews. Amsterdam: Elsevier/North-Holland Biomedical press; 1978. p. 65–125.
Ponferrada VG, Mauck BS, Wooley DP. The envelope glycoprotein of human endogenous retrovirus HERV-W induces cellular resistance to spleen necrosis virus. Arch Virol. 2003;148:659–75.
Potgens AJG, Drewlo S, Kokozidou M, Kaufmann P. Syncytin: the major regulator of trophoblast fusion? Recent developments and hypotheses on its action. Hum Reprod Update. 2004;10:487–96.
Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C. The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol. 2006;17:254–63.
Gordon-Alonso M, Yañez-Mó M, Barreiro O, Álvarez S, Muñoz-Fernández MÁ, Valenzuela-Fernández A, et al. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177:5129–37.
Ethical statement
All participating persons gave their informed consent prior inclusion in the study. The Danish Ethical committee approval was obtained before the study was initiated (J. nr H-B-2008-150).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Membrane fusion is an important part of fertilization. Here we present, for the first time, the presence of a true fusogen Syncytin-1 and its receptor on human gametes.
This work was conducted at The Fertility Clinic, Rigshospitalet and at the Faculty of Life Science, University of Copenhagen
Rights and permissions
About this article
Cite this article
Bjerregaard, B., Lemmen, J.G., Petersen, M.R. et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet 31, 533–539 (2014). https://doi.org/10.1007/s10815-014-0224-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0224-1