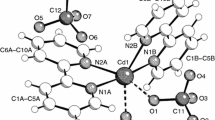
Dinonyldithiophosphoric acid (DNDTPA) was prepared by adding phosphorus pentasulfide to nonyl alcohol. The lead dinonyl dithiophosphate complex Pb(DNDTP) 2 was prepared by mixing a solution of lead(II) with a solution of DNDTPA in ethanol at room temperature. The recovered Pb(DNDTP) 2 was crystallized in ethanol, and fi ne colorless needles were obtained. The complex was characterised by elemental analysis and IR, UV–vis and atomic absorption spectroscopy. The thermal behavior of Pb(DNDTP) 2 was investigated by thermogravimetric analysis under a nitrogen atmosphere. Removal of Pb(II) from aqueous media by DNDTPA solutions was investigated. The optimum conditions for extraction, such as the organic solvent, pH of the aqueous phase, time of equilibration, concentration, and effect of anions, were investigated. It was found that DNDTPA is an effective substance for removing Pb(II) from aqueous solution.
Similar content being viewed by others
References
G. Cote and D. Bauer, Rev. Inorg. Chem., 10, 121–144 (1989).
I. Haiduc, Coord. Chem. Rev., 158, 325–358 (1997).
N. Biricik and B. Gümgüm, Thermochim. Acta, 417, 43–45 (2004).
P. G. Harrison, Wear, 123, 117–118 (1988).
P. G. Harrison, M. G. Begley, T. Kikabhai, and A. T. Steel, J. Chem. Soc. Dalton Trans., 1, 2443–2448 (1989).
K. H. Ebert, H. J. Breunigh, C. Silvestru, I. Stefan, and I. Haiduc, Inorg. Chim. Acta, 33, 1695–1699 (1994).
B. Menoyo, R. Benito, and M. P. Elizalde, Solvent Extr. Ion Exch., 14, 69–88 (1996).
A. P. Argekar and A. K. Shetty, Talanta, 45, 909–915 (1998).
J. O. Esalah, M. E. Weber, and J. H. Vera, Sep. Purif. Technol., 18, 25–36 (2000).
R. A. Varga, C. Silvestru, and I. Haiduc, Synth. React. Inorg. Met.-Org. Chem., 30, 485–498 (2000).
S. Al-Malaika, M. Coker, and G. Scott, Polym. Degrad. Stab., 22, 147–159 (1988).
R. Schumacher and H. Zinke, Tribol. Int., 30, 199–208 (1997).
C. Keromest, J. P. Durand, M. Born, P. Gateau, M. Tessier, and E. Marechal, Rev. Inst. Petrol., 52, 35–44 (1997).
D. R. Amstrong, E. S. Ferrari, K. J. Roberts, and D. Adams, Wear, 208, 138–146 (1997).
H. Sabik and R. Jeannot, J. Chromatogr. A, 879, 73–82 (2000).
A. Aguera, M. Contreras, and A. R. Fernandezalba, J. Chromatogr. A, 655, 293–300 (1993).
S. Lacorte, S. B. Lartiges, P. Garrigues, and D. Barcelo, Environ. Sci. Technol., 29, 431–438 (1995).
I. Persson, J. Coord. Chem., 32, 261–342 (1994).
X. Ying and Z. Fang, J. Hazard. Mater. B, 137, 1636–1642 (2006).
Y. Xu, Y. Chen, and Y. Feng, Environ. Technol., 34, 1411–1419 (2013).
T. H. Handley, Anal. Chem., 35, 991–995 (1963).
T. H. Handley and J. A. Dean, Anal. Chem., 43, 1312–1315 (1963).
S. Wingefors, Acta Chem. Scand., 34, 289–296 (1980).
V. F. Toropova, A. R. Garifzyanov, and I. E. Panfilova, Talanta, 34, 211–214 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 82, No. 3, pp. 465–468, May–June, 2015.
Rights and permissions
About this article
Cite this article
Gümgüm, H.B., Biricik, N. Spectroscopic and Thermogravimetric Characterization of Pb(II) Dinonyldithiophosphate: Removal of Pb(II) from Aqueous Solution. J Appl Spectrosc 82, 475–478 (2015). https://doi.org/10.1007/s10812-015-0133-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-015-0133-9