Abstract
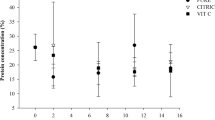
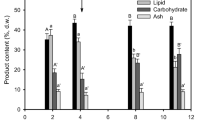
The aim of this paper is to solve problems that may arise in microalgae cultivation facilities at a hatchery in such a way that its activities are not disrupted. Thus, the effect of storage conditions (time, initial biomass concentration, presence or absence of light, addition of preservatives and conservation atmospheres) on cell viability, evolution of sample concentration, contamination, fatty acid and pigment content for long-term preservation of Nannochloropsis gaditana biomass has been studied. The polyunsaturated fatty acid content was maintained and the eicosapentaenoic acid (EPA, 20:5n3) content was kept stable at 3.6 % (d.w.). The total pigments content was also preserved at 0.85 % (d.w.), what allowed maintaining the antioxidant activity of samples. The results show that samples preserved both as concentrated inocula ready to run a photobioreactor, at an initial biomass (N. gaditana) concentration of 5 g L−1, and as pastes to be used as live food if any incident happens in a hatchery, at 150 g L−1, can be stored for up to 4 months at 4 °C under nitrogen or air atmosphere of conservation maintaining the cell viability and the sample concentration stable and at low incident irradiance (35 μmol photons m−2 s−1). This allows the photosynthetic activity of cells to be preserved in the case of samples at 5 g L−1. The bacterial load was kept at low level by the regular addition of preservative agents.







Similar content being viewed by others
References
Alías CB, García-Malea López MC, Acién-Fernández FG, Fernández-Sevilla JM, García-Sánchez JL, Molina-Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87:723–733
Aragão C, Conceição L, Dinis M, Fyhn HJ (2004) Amino acid pools of rotifers and Artemia under different conditions: nutritional implications for fish larvae. Aquaculture 234:429–445
Babarro JMF, Fernández-Reiriz MJ, Labarta U (2001) Influence of preservation techniques and freezing storage time on biochemical composition and spectrum of fatty acids of Isochrysis galbana clone T-ISO. Aquacult Res 32:565–572
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Camacho-Rodríguez J, Cerón-García MC, González-López CV, Fernández-Sevilla JM, Contreras-Gómez A, Molina-Grima E (2013) A low-cost culture medium for the production of Nannochloropsis gaditana biomass optimized for aquaculture. Bioresour Technol 144:57–66
Cerón MC, García-Malea MC, Rivas J, Acién FG, Fernández JM, Del Río E, Guerrero MG, Molina E (2007) Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biotechnol 74:1112–1119
Chini Zittelli G, Rodolfi L, Ponis E, Tredici MR (2003) Cold preservation and use of microalgae aquaculture feed. Abstract of the 5th European Workshop on Biotechnology of Microalgae, 23–24 June 2003, Bergholz-Rehbrücke
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Cordero B, Voltolina D (1997) Viability of mass algal cultures preserved by freezing and freeze-drying. Aquacult Eng 16:205–211
Day JG, Fleck RA, Benson EE (2000) Cryopreservation-recalcitrance in microalgae: novel approaches to identify and avoid cryo-injury. J Appl Phycol 12:369–377
Dorsey J, Yentsch CM, Mayo S, McKenna C (1989) Rapid analytical technique for the assessment of cell metabolic activity in marine microalgae. Cytometry 10:622–628
FAO Fisheries Technical Paper 361 (1996) In: Lavens P, Sorgeloos P (eds) Manual on the production and use of live food for aquaculture, 1st edn. FAO, Rome, 295 pp
González-López CV, Cerón-García MC, Fernández-Sevilla JM, González-Céspedes AM, Camacho-Rodríguez J, Molina-Grima E (2013) Medium recycling for Nannochloropsis gaditana cultures for aquaculture. Bioresour Technol 129:430–438
Gouveia L, Empis J (2003) Relative stabilities of microalgal carotenoids in microalgal extracts, biomass and fish feed: effect of storage conditions. Innov Food Sci Emerg Technol 4:227–233
Guermazi W, Sellami-Kammoun A, Ellormi J, Drira Z, Aleya L, Marangoni R, Ayadi H, Maalej S (2010) Microalgal cryo-preservation using dimethyl sulfoxide (Me2SO) coupled with two freezing protocols: influence on the fatty acid profile. J Therm Biol 35:175–181
Hansmann E (1973) Pigment analysis. In: Stein JR (ed) Handbook of phycological methods, culture methods and growth measurements. Cambridge University, Cambridge, pp 359–368
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkes L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs—a summary. Aquac Res 31:637–659
Heras H, Kean-Howie J, Ackman RG (1994) The potential use of lipid microspheres as nutritional supplements for adult Ostrea edulis. Aquaculture 123:309–322
Huis in’t Veld JHJ (1996) Microbial and biochemical spoilage of foods: an overview. Int J Food Microbiol 33:1–18
Jones DA, Kurmaly K, Arshard A (1987) Penaeid shrimp hatchery trials using microencapsulated diets. Aquaculture 64:133–146
Kanazawa A, Teshima S-I, Ono K (1979) Relationship between essential fatty acid requirements of aquatic animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp Biochem Physiol 63B:295–298
Molina-Grima E, Sánchez-Pérez JA, Farcía-Camacho F, Acién-Fernández FG, López-Alonso D, Segura del Castillo CI (1994) Preservation of the marine microalga, Isochrysis galbana: influence on the fatty acid profile. Aquaculture 123:377–385
Montaini E, Chini-Zittelli G, Tredici MR, Molina-Grima E, Fernández-Sevilla JM, Sánchez-Pérez JA (1995) Long-term preservation of Tetraselmis suecica: influence of storage on viability and fatty acid profile. Aquaculture 134:81–90
Morais S, Koven W, Rønnestad I, Dinis MT, Conceição LEC (2005) Dietary protein: lipid ratio and lipid nature affects fatty acid absorption and metabolism in a teleost larva. Br J Nutr 93:813–820
Nell JA, Diemar JA, Heasman MP (1996) Food value of live yeasts and dry yeast-based diets fed to Sydney rock oyster Saccostrea commercialis spat. Aquaculture 145:235–243
Nunes M, Pereira A, Ferreira JF, Yasumaru F (2009) Evaluation of the microalgae paste viability produced in a mollusk hatchery in southern Brazil. J World Aquacult Soc 40:87–94
Parsons TR, Strickland JDH (1973) Pigment analysis. In: Stein JR (ed) Handbook of phycological methods, culture methods and growth measurements, 1st edn. London, UK, pp 359–368
Piña P, Voltolina D, Nieves M, Robles M (2006) Survival, development and growth of the pacific white shrimp Litopenaeus vannamei protozoea larvae, fed with monoalgal and mixed diets. Aquaculture 253:523–530
Ponis E, Parisi G, Chini-Zittelli G, Lavista F, Robert R, Tredici MR (2008) Pavlova lutheri: production, preservation and use as food for Crassostrea gigas larvae. Aquaculture 282:97–103
Prado R, Rioboo C, Herrero C, Suárez-Bregua P, Cid A (2012) Flow cytometric analysis to evaluate physiological alterations in herbicide-exposed Chlamydomonas moewusii cells. Ecotoxicology 21:409–420
Robinson CB, Samocha TM, Fox JM, Gandy RL, McKee DA (2005) The use of inert artificial commercial food sources as replacements of traditional live food items in the culture of larval shrimp, Farfantepenaeus aztecus. Aquaculture 245:135–147
Rocha JMS, García JEC, Henriques MHF (2003) Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol Eng 20:237–242
Rodríguez-Ruíz J, Belarbi E, Sánchez JLG, Alonso DL (1998) Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol Tech 12:689–691
Ryckebosch E, Muylaert K, Eeckhout M, Ruyssen T, Foubert I (2011) Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J Agric Food Chem 59:11063–11069
San Pedro A, González-López CV, Acién FG, Molina-Grima E (2014) Outdoor pilot-scale production of Nannochloropsis gaditana: influence of culture parameters and lipid production rates in tubular photobioreactors. Bioresour Technol 169:667–676
San Pedro A, González-López CV, Acién FG, Molina-Grima E (2015) Outdoor pilot production of Nannochloropsis gaditana: influence of culture parameters and lipid production rates in raceway ponds. Algal Res 8:205–213
Tzovenis I, Triantaphyllidis G, Naihong X, Chatzinikolaou E, Papadopoulou K, Xouri G, Tafas T (2004) Cryopreservation of marine microalgae and potential toxicity of cryoprotectants to the primary steps of the aquacultural food chain. Aquaculture 230:457–473
Verdelho Vieira V (2014) Microalgae for fuel and beyond: future trends. International Society for Applied Phycology Newsletter 5–17
Vizcaíno AJ, López G, Sáez MI, Jiménez JA, Barros A, Hidalgo L, Camacho-Rodríguez J, Martínez TF, Cerón-García MC, Alarcón FJ (2014) Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 431:34–43
Welladsen H, Kent M, Mangott A, Li Y (2014) Shelf-life assessment of microalgae concentrates: effect of cold preservation on microalgal nutrition profiles. Aquaculture 430:241–247
Xiao X, Han Z-Y, Chen Y-X, Liang X-Q, Li H, Qian Y-C (2011) Optimization of FDA-PI method using flow cytometry to measure metabolic activity of the cyanobacteria, Microcystis aeruginosa. Phys Chem Earth 36:424–429
Acknowledgments
This research was supported by the General Secretariat of Universities, Research and Technology of the Andalusian Government (AGR-5334) and was co-financed by FEDER funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camacho-Rodríguez, J., Cerón-García, M.C., Macías-Sánchez, M.D. et al. Long-term preservation of concentrated Nannochloropsis gaditana cultures for use in aquaculture. J Appl Phycol 28, 299–312 (2016). https://doi.org/10.1007/s10811-015-0572-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0572-y