Abstract
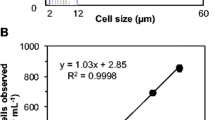
Determining the fraction of viable cells in algal cultures is critical to improve the understanding and control of algal microbiology, ecology, and biotechnology. Whereas current techniques for algal viability determination can be rather cumbersome, this paper describes a new assay that enables the rapid quantification of algal viability using only spectrophotometric measurements. This technique, henceforth named colorimetric assay of viability for algae (CAVA), relies on the selective adsorption of erythrosine by non-viable cells and was validated on the algae species Chlamydomonas reinhardtii and Chlorella vulgaris. The results obtained by the CAVA test were in good agreement with the in situ measurement of oxygen production rates. In addition, the CAVA test was shown to quantify the viability of algal samples regardless of the cause of death (heating, UV irradiation, or H2O2 exposure). The CAVA technique has therefore the potential to offer a fast and universal approach to measure the viability of algal samples.





Similar content being viewed by others
References
Allen MM, Smith AJ (1969) Nitrogen chlorosis in blue-green algae. Arch Mikrobiol 69:114–120
Andersen RA, Berges JA, Harisson PJ, Watanabe MM (2005) Appendix A-Recipes for freshwater and seawater media. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, pp 429–530
Alías CB, García-Malea López MC, Acién Fernández FG, Fernández Sevilla JM, García Sánchez JL, Molina Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87:723–733
Béchet Q, Chambonnière P, Shilton A, Guizard G, Guieysse B (2014) Algal productivity modeling: a step toward accurate assessments of full-scale algal cultivation. Biotechnol Bioeng. doi:10.1002/bit.25517
Béchet Q, Shilton A, Fringer O, Munoz R, Guieysse B (2010) Mechanistic modelling of broth temperature in outdoor photobioreactors. Environ Sci Technol 44:2197–2203
Berglund DL, Taffs RE, Robertson NP (1987) A rapid analytical technique for flow cytometric analysis of cell viability using Calcofluor White M2R. Cytometry 8:421–426
Breeuwer P, Abee T (2000) Assessment of viability of microorganisms employing fluorescence techniques. Int J Food Microbiol 55:193–200
Capasso JM, Cossío BR, Berl T, Rivard CJ, Jiménez C (2003) A colorimetric assay for determination of cell viability in algal cultures. Biomol Eng 20:133–138
Clarke JM, Gillings MR, Altavilla N, Beattie AJ (2001) Potential problems with fluorescein diacetate assays of cell viability when testing natural products for antimicrobial activity. J Biol Methods 46:261–267
Drábková M, Admiraal W, Marsálek B (2007) Combined exposure to hydrogen peroxide and lights selective effects on cyanobacteria, green algae, and diatoms. Environ Sci Technol 41:309–314
Garvey M, Moriceau B, Passow U (2007) Applicability of the FDA assay to determine the viability of marine phytoplankton under different environmental conditions. Mar Ecol Prog Ser 352:17–26
Garza DR, Suttle CA (1998) The effect of cyanophages on the mortality of Synechococcus spp. and selection for UV resistant viral communities. Microb Ecol 36:281–292
Gilbert F, Galgani F, Cadiou Y (1992) Rapid assessment of metabolic activity in marine microalgae: application in ecotoxicological tests and evaluation of water quality. Mar Biol 112:199–205
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A 54:1665–1669
Gudin C, Chaumont D (1991) Cell fragility—the key problem of microalgae mass production in closed photobioreactors. Bioresour Technol 38:145–151
Gupta VK, Mittal A, Kurup L, Mittal J (2006) Adsorption of a hazardous dye, erythrosine, over hen feathers. J Colloid Interface Sci 304:52–57
Häder DP, Kumar HD, Smith RC, Worrest RC (2007) Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 6:267–285
Hwang SW, Horneland W (1965) Survival of algal cultures after freezing by controlled and uncontrolled cooling. Cryobiology 1:305–311
Imase M, Ohko Y, Takeuchi M, Hanada S (2013) Estimating the viability of Chlorella exposed to oxidative stresses based around photocatalysis. Int Biodeterior Biodegrad 78:1–6
Jain R, Sikarwar S (2009) Adsorptive removal of Erythrosine dye onto activated low cost de-oiled mustard. J Hazard Mater 164:627–633
James GO, Hocart CH, Hillier W, Chen H, Kordbacheh F, Price GD, Djordjevic MA (2011) Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresour Technol 102:3343–3351
Low KS, Lee CK, Toh BL (1994) Binding of basic dyes by the algae, Chara aspera. Pertanika J Sci Technol 2(1):85–92
Madigan MT, Martinko JM (2006) Brock—biology of microorganisms, 11th edn. Pearson Prentice Hall, Upper Saddle River, 07458
Markelova AG, Vladimirova MG, Kuptsova ES (2000) A comparison of cytochemical methods for the rapid evaluation of microalgal viability. Russ J Plant Physiol 47:815–819
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Mittal A, Mittal J, Kurup L, Singh AK (2006) Process development for the removal and recovery of hazardous dye erythrosine from wastewater by waste materials—bottom ash and de-oiled soya as adsorbents. J Hazard Mater B 138:95–105
Pouneva I (1997) Evaluation of algal culture viability and physiological state by fluorescent microscopic methods. Bulg J Plant Physiol 23:67–76
Saga N, Machiguchi Y, Sanbonsuga Y (1987) Application of staining for determining viability of cultured algal cells. Bull Hokkaido Reg Fish Res Lab 51:39–44
Sato M, Murata Y, Mizusawa M, Iwahashi H, Oka S (2004). A simple and rapid dual fluorescence viability assay for Microalgae. Microbiol Cult Coll Dec. 20(2):53–59
Schulze K, López DA, Tillich UM, Frohme M (2011) A simple viability analysis for unicellular cyanobacteria using a new autofluorescence assay, automated microscopy, and ImageJ. BMC Biotechnol 11:118
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Steinberg MK, Lemieux EJ, Drake LA (2011) Determining the viability of marine protists using a combination of vital, fluorescent stains. Mar Biol 158:1431–1437
Venkata Mohan S, Ramanaiah SV, Sarma PN (2008) Biosorption of direct azo dye from aqueous phase onto Spirogyra sp. I02: evaluation of kinetics and mechanistic aspects. Biochem Eng J 38:61–69
Acknowledgments
Dr. Mike Packer, Cawthron Institute (New Zealand), is kindly acknowledged for providing axenic cultures of C. reinhardtii. Cyril Breton, Ecole Centrale Paris (France), and Laura Etienne, Massey University (New Zealand), are also acknowledged for preliminary experimental work during the technique development. Maxence Plouviez, Massey University (New Zealand), is also thanked for his help all along the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 651 kb)
Rights and permissions
About this article
Cite this article
Béchet, Q., Feurgard, I., Guieysse, B. et al. The colorimetric assay of viability for algae (CAVA): a fast and accurate technique. J Appl Phycol 27, 2289–2297 (2015). https://doi.org/10.1007/s10811-014-0508-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0508-y