Abstract
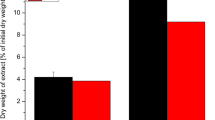
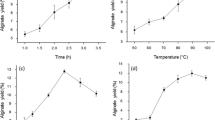
A new analysis method for alginate quantification in the brown alga, Saccharina japonica, was developed and evaluated. In this method, (1) alginate was treated with Na2CO3; (2) the alginate-derived compound in the hydrolysate was analyzed using high-performance liquid chromatography (HPLC); and then, (3) one of the HPLC peaks was selected and used to establish the standard calibration curve to estimate the intact alginate content in the raw material. The results obtained using the new method were verified by the Kennedy and Bradshaw method, which confirmed that the new method can be an effective method for the estimation of the alginate content in S. japonica. An experimental equation was developed to estimate the alginic acid concentration in the hydrolysate obtained from Na2CO3 treatment of the model compound (Sigma Na-alginate) at various reaction conditions on the basis of the correlation between estimated alginic acid contents and combined severity factors (CSF). The statistical analysis confirmed that the equation gave consistent results, i.e., approximately 81 % of the test groups lie within <±10 % standard deviation of the mean.




Similar content being viewed by others
References
Adams JM, Gallagher JA, Donnison IS (2009) Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol 21:569–574
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Chum HL, Johnson DK, Black SK, Overend RP (1990) Pretreatment-catalyst effects and the combined severity parameter. Appl Biochem Biotechnol 24–25:1–14
Committee on Specifications, Food Chemical Codex of the Committee on Food Protection National Research Council (1972) Food chemicals codex, 2nd edn. National Academy of Sciences, Washington, D.C., 863 p
Dubois M, Gillus KA, Hamilton JK, Robers PA, Smith F (1956) Colorimetric method for sugars and related substance. Anal Chem 28:350–356
Goh CS, Lee KT (2010) A visionary and conceptual macroalgae-based third-generation bioethanol (TGB) biorefinary in Sabah, Malaysia as an underlay for renewable and sustainable development. Renew Sust Energ Rev 14:842–848
Graham HD (1969) Determination of alginate in dairy products. J Dairy Sci 52:445–448
Guo G, Chen WH, Chen WH, Men LC, Hwang WS (2008) Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresour Technol 99:6046–6053
Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G (2006) Bio-ethanol the fuel of tomorrow from the residues of today. Trends Biotechnol 24:549–556
Ueda R, Ogushi Y, Hirano A, Samejima Y, Hon-Nami K, Kunito S (1998) Ethanol production from carbon dioxide by fermentative microalgae. Stud Surf Sci Catal 114:657–660
Honya M, Kinoshita T, Ishikawa M, Mori H, Nisizawa K (1993) Monthly determination of alginate, M/G ratio, mannitol, and minerals in cultivated L. japonica. Nippon Suisan Gakkaishi 59:295–299
Horn SJ, Aasen IM, Qstgaard K (2000a) Ethanol production from seaweed extract. J Ind Microbiol Biotechnol 25:249–254
Horn SJ, Aasen IM, Qstgaard K (2000b) Production of ethanol from mannitol by Zymobacter palmae. J Ind Microbiol Biotechnol 24:51–57
Ishikawa K, Ueyama Y, Mano T, Koyama T, Suzuki K, Matsumura T (1999) Self-setting barrier membrane for guided tissue regeneration method: initial evaluation of alginate membrane made with sodium alginate and calcium chloride aqueous solutions. J Biomed Mater Res 47:111–115
Jeong TS, Oh KK (2011) Optimization of fermentable sugar production from rape straw through hydrothermal acid pretreatment. Bioresour Technol 102:9261–9266
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol 102:186–193
Kennedy JF, Bradshaw IJ (1984) A rapid method for the assay of alginates in solution using polyhexamethylenebiguanidinium chloride. Br Polym J 16:95–101
Kloareg B, Quatrano RS (1988) Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Annu Rev 26:259–315
Kulseng B, Skjsk-Braek G, Ryan L, Andersson A, King A, Faxbang A, Espevik T (1999) Transplantation of alginate microcapsules: generation of antibodies against alginates and encapsulated porcine islet-like cell clusters. Transplantation 67:978–984
Lee JW, Jeffries TW (2011) Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour Technol 102:5884–5890
Mattiason B (1983) In the immobilized cells and cellular organelles. CRC Press, Boca Raton, pp 3–35
McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical Paper 441
Meinita MDN, Marhaeni B, Winanto T, Jeong G-T, Khan MNA, Hong Y-K (2013) Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol 25:1957–1961
Overend RP, Chornet E (1987) Fractionation of lignocellulosics by steam/aqueous pretreatments. Phil Trans R Soc Lond A321:523–536
Panagiotopoulos IA, Bakker RR, de Vrije T, Koukios EG (2011) Effect of pretreatment severity on the conversion of barley straw to fermentable substrates and the release of inhibitory compounds. Bioresour Technol 102:1120–11211
Perez R, Kaas R, Campello F, Arbault S, Barbaroux O (1992) La culture des algues marines dans le monde. IFREMER, Plouzane, p 163
Prasad S, Singh A, Jain N, Joshi HC (2007a) Ethanol production from sweet sorghum syrup for utilization as automotive fuel in India. Energy Fuels 21:2415–2420
Prasad S, Singh A, Joshi HC (2007b) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour Conserv 50:1–39
Saha BC, Cotta MA (2007) Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzym Microb Technol 41:528–532
Singh A, Pant D, Korres NE, Nizami AS, Prasad S, Murphy JD (2010) Key issues in life cycle assessment of ethanol production from lignocellulosic biomass: challenges and perspectives. Bioresour Technol 101:5003–5012
Suzuki Y, Tanihara M, Ohnishi K, Suzuki K, Endo K, Nishimura Y (1999) Cat peripheral nerve regeneration across 50 mm gap repaired with a novel nerve guide composed of freeze-dried alginate gel. Neurosci Lett 259:75–78
Vauchel P, Kaas R, Arhaliass A, Baron R, Legrand J (2008) A new process for extracting alginate from Laminaria digita: reactive extraction. Food Bioproc Technol 1:297–300
Vauchel P, Leroux R, Kaas R, Arhaliass A, Baron R, Legrand J (2009) Kinetics modeling of alginate alkaline extraction from Laminaria digitata. Bioresour Technol 100:1291–1296
Wagner W (1963) Studien über die Naphthoresorcin-Reaktion von Tollens : Zur Bestimmung von freier Uronsäure neben Uronosiden, Polyuronosiden und neben anderen Kohlenhydraten. Anal Chim Acta 29:227–239
Wi SG, Kim HJ, Mahadevan SA, Yang DJ, Bae HJ (2009) The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour Technol 100:6658–6660
Yeon JH, Lee SE, Choi WY, Kang DH, Lee HY, Jung KH (2011) Repeated-batch operation of surface-aerated fermenter for bioethanol production from the hydrosate of seaweed Sargassum sagamianum. J Microbiol Biotechnol 21:323–331
Acknowledgments
This work was financially supported by the Ministry of Oceans and Fisheries (contract no. 20131039449).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.H., Choi, W.I., Kim, Y.S. et al. A novel alginate quantification method using high-performance liquid chromatography (HPLC) for pretreatment of Saccharina japonica . J Appl Phycol 27, 511–518 (2015). https://doi.org/10.1007/s10811-014-0298-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0298-2