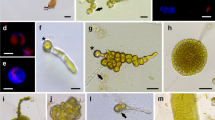
Abstract
The tropical agarophyte Gracilaria changii has been much researched and documented by the Algae Research Laboratory, University of Malaya, especially with regards to its potential as a seaweed bioreactor for valuable compounds. Protoplast regeneration of this seaweed was developed following the optimization of protoplast isolation protocol. Effect of the concentration and combination of isolating enzymes, incubation period, temperature, enzyme solution pH, tissue source on the protoplast yields were used to optimize the isolation protocol. The enzyme mixture with 4% w/v cellulase Onozuka R-10, 2% w/v macerozyme R-10 and 1 unit mL-1 agarase was found to produce the highest yield of protoplast at 28°C and 3 h incubation period. Thallus tips gave higher yields of protoplasts than middle segments. Freshly isolated G. changii protoplasts were cultured in MES medium. Regeneration of protoplast cell walls after 24 h was confirmed by calcofluor white M2R staining under UV fluorescence microscopy. The protoplasts with regenerated cell walls then underwent a series of cell division to produce callus-like cell masses in MES medium. Following this, juvenile plants of G. changii were obtained.











Similar content being viewed by others
References
Araki T, Hayakawa M, Tamaru Y, Yoshimatsu K, Morishita T (1994) Isolation and regeneration of haploid protoplasts from Bangia atropurpurea (Rhodophyta) with marine bacterial enzymes. J Phycol 30:1040–1046
Araki T, Lu Z, Morishita T (1998) Optimization of parameters for isolation of protplasts from Gracilaria verrucosa (Rhodophyta). J Mar Biotechnol 6:193–197
Bellanger F, Verdus MC, Henocq V, Christiaen (1990) Determination of the composition of the fibrillar part of Gracilaria verrucosa (Gracilariales, Rhodopyta) cell wall in order to prepare protoplasts. Hydrobiologia 204/205:527–531
Björk M, Ekman P, Wallin A, Pedersén M (1990) Effects of growth rate and other factors on protoplast yield from four species of Gracilaria (Rhodophyta). Bot Mar 33:433–439
Butler DM, Evans LV, Kloareg B (1990) Isolation of protoplasts from marine macroalgae. In: Akatsuka I (ed) Introduction to applied phycology. SPB Academic, Netherlands, pp 647–668
Chan CX, Teo SS, Ho CL, Rofima Yasmin O, Phang SM (2004) Optimisation of RNA extraction for Gracilaria changii (Gracilariales, Rhodophyta). J Appl Phycol 16:297–301
Chen YC, Chiang YM (1994) Development of protoplast from Grateloupia sparsa and Grateloupia filicina (Halymeniaceae, Rhodophyta). Bot Mar 37:361–366
Chen LC-M, Craigie JS, Xie ZK (1994) Protoplast production from Porphyra linearis a simplified agarase procedure capable of commercial application. J Appl Phycol 6:35–39
Cheney DP, Mar E, Saga N, Meer J (1986) Protoplast isolation and cell division in the agar-producing seaweed Gracilaria (Rhodophyta). J Phycol 22:238–243
Chu J, Liu W, Zhang Z (1998) A preliminary study on the isolation and cultivation of protoplast from G. verrucosa. Mar Sci Bull 17(6):17–20 (In Chinese with English abstract)
Chu WL, Norazmi M, Phang SM (2003) Fatty acid composition of some Malaysian seaweeds. Malays J Sci 22(2):21–27
Cocking EC (2000) Turning point article plant protoplasts. In Vitro Cell Dev Biol Plant 36:77–82
Collén J, Roeder V, Rousvoal S, Collin O, Kloareg B, Boyen C (2006) An expressed sequence tag analysis of thallus and regenerating protoplasts of Condrus crispus (Gigartinales, Rhodophyceae). J Phycol 42:104–112
Collin HA, Edwards S (1998) Protoplast culture. In: Rickwood D, Howe C (eds) Plant cell culture. The introduction to biotechniques series. Bios Scientific, Oxford UK, pp 71–81
Compton ME, Saunders JA, Veilleux RE (2000) Use of protoplast for plant improvement. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercise. CRC, USA, pp 249–261
Coury DA, Naganuma T, Polne-Fuller M, Gibor A (1993) Protoplasts of Gelidium robustum (Rhodophyta). Hydrobiologia 260/261:421–427
Dipakkore S, Reddy CRK, Jha B (2005) Production and seeding of protoplast of Porphyra okhaensis (Bangials, Rhodophyta) in laboratory culture. J Appl Phycol 17:331–337
Evans DA, Bravo JE (1983) Protoplast isolation and culture. In: Evans DA, Sharp WR, Ammirato P, Yamada Y (eds) Techniques gor propagation and breeding. Handbook of plant cell culture, vol 1. Technique for propagation and breeding. Macmillan, New York, USA, pp 124–176
Gan SY, Qin S, Rofina YO, Yu D, Phang SM (2003) Transient expression of lacZ in particle bombarded Gracilaria changii (Gracilariales, Rhodophyta). J Appl Phycol 15:315–353
Gan SY, Rofina YO, Qin S, Phang SM (2006) Crop improvement in seaweed cultivation. In: Phang SM, Crichery A, Ang P (eds) Advances in seaweed cultivation and utilization in Asia. University of Malaya Maritime Research Centre, Kuala Lumpur, Malaysia, pp 81–103
Graham LE, Wilcox LW (2000) Red algae. In: Algae. Prentice-Hall, Upper Saddle River, NJ, pp 343–396
Guillard RRL (1973) Division rates. In: Stein JR (ed) Culture methods and growth measurements. Handbook of Phycological Methods. Cambridge University Press, London, pp 289–311
Hunter CF (1994) Protoplast and haploid cultures. In: Hunter CF (ed) In vitro cultivation of plant cells. Butterworth-Heinemann, Oxford, pp 87–112
Kito H, Kunimoto M, Kamanishi Y, Mizukami Y (1998) Protoplast fusion between Monostroma nitidum and Porphyra yezoensis and subsequent growth of hybrid plants. J Appl Phycol 10:15–21
Kumar GRK, Addepalli MK, Reddy CRK (1999) Regeneration of the thallus of Monostroma oxyspermum (Chlorophyta) from protoplasts in axenic culture. Phycologia 38:503–507
Kumar GR, Reddy CKR, Jha B (2007) Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J Appl Phycol 19:15–25
Lim PE, Phang SM (2004) Gracilaria species (Gracilariales, Rhodophyta) of Malaysia including two new recods. Malays J Sci 23(2):71–80
Liu QY, Chen LC-M, Taylor ARA (1992) Ultrastructure of cell wall regeneration by isolated protoplasts of Palmaria palmata (Rhodophyta). Bot Mar 35:21–33
Marinho-Soriano, Silva ETSF, Moreira WSC (2001) Seasonal variation in the biomass and agar yield from Gracilaria cervicornis and Hydropuntia cornea from Brazil. Bioresour Technol 77(2):115–120
McHugh DJ (2003) A guide to the seaweed industry. FAO Fisheries Technical Paper No 441. FAO, Rome, p 105
Norziah MH, Chio YC (2000) Nutritional composition of edible seaweed Gracilaria changii. Food Chem 68:69–76
Ochatt SJ, Power JB (1992) Plant regeneration from cultured protoplasts of higher plants. In: Fowler MW, Warren GS (eds) Plant biotechnology. Comprehensive Biotechnology, Second supplement. Pergamon, Oxford, pp 99–127
Phang SM (2006) Seaweed resources in Malaysia: current status and future prospects. Aquat Ecosyst Health Manag 9:185–202
Phang SM, Shaharuddin S, Noraishah H, Sasekumar A (1996) Studies on Gracilaria changii (Gracilariales, Rhodophyta) from Malaysian mangroves. Hydrobiologia 326/327:347–352
Pinchetti JLG, Björk M, Pedersén M, Reina GG (1993) Factors affecting protoplast yield of the carrageenophyte Solieria filiformis (Gigartinales, Rhodophyta). Plant Cell Rep 12:541–545
Reddy CRK, Dipakkore S, Kumar R, Jha B, Cheney DP, Fujita Y (2006) An improved enzyme preparation for rapid mass production of protoplast as seed stock for aquaculture of macrophytic marine green algae. Aquaculture 260:290–297
Saga N, Kudo T (1989) Isolation and culture of protoplast from the marine green alga Monostroma angicava. J Appl Phycol 1:25–30
Saga N, Polne-Fuller M, Gibor A (1986) Protoplast from seaweed: production and fusion. Beih Nova Hedwig 83:37–43
Salvador RC, Serrano AE (2005) Isolation of protoplasts from tissue fragments of Philippine cultivars of Kappaphycus alvarezii (Solieriaceae, Rhodophyta). J Appl Phycol 17:15–22
Santelices B, Correa JA, Hormazábal M, Flores V (2003) Contact responses between spores and sporelings of different species, karyological phases and cystocarps of coalescing Rhodophyta. Mar Biol 143:381–392
Tan EL (1999) Tissue culture and protoplast isolation of Gracilaria changii (Rhodophyta). Dissertation, University of Malaya
Teo SS, Ho CL, Teoh S, Lee WW, Tee JM, Raha AR, Phang SM (2007) Analyses of expressed sequence tags from an agarophyte, Gracilaria changii (Gracilariales, Rhodophyta). Eur J Phycol 42:41–46
Wong WH, Goh SH, Phang SM (1994) Antibacterial properties of Malaysian seaweeds. In: Phang SM, Lee YK, Borowitzka MA, Whitton BA (eds) Algal biotechnology in the Asia-Pacific region. University of Malaya, Kuala Lumpur, pp 75–81
Wong PF, Tan LJ, Nawi H, AbuBakar S (2006) Proteomics of the red alga, Gracilaria changii (Gracilariales, Rhodophyta). J Phycol 42:113–120
Yan XH, Wang SJ (1993) Regeneration of whole plants from Gracilaria asiatica Chang et Xia protoplasts (Gracilariaceae, Rhodophyta). Hydrobiologia 260/261:429–436
Zhang QQ (1991) Studies on the isolation, culture and regeneration of protoplasts in Chondrus ocellatus Holm. J Qingdao Oceanol Univ 21:52–62
Acknowledgments
This research was funded by the Ministry of Science, Technology and Innovation, Malaysia (Grant No: 39-02-03-9002/Oracle 8301902; 12-02-03-2028/Oracle 8422028), University of Malaya Short-term Research Grant No. F0140/2002D, F0133/2004A, F0233/2004D and P0114/2006A. Ms Yeong conducted this research under a PASCA scholarship from the University of Malaya. The authors gratefully acknowledge the following for their advice and kind assistance: Professor He Peimin, Dr. Gan Sook Yee, Dr. Tan Eng Lai, Professor Qin Song and Dr. Jiang Peng.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeong, HY., Khalid, N. & Phang, SM. Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). J Appl Phycol 20, 641–651 (2008). https://doi.org/10.1007/s10811-007-9249-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-007-9249-5