Abstract
Recent studies have suggested that sensory processing atypicalities may share genetic influences with autism spectrum disorder (ASD). To further investigate this, the adolescent/adult sensory profile (AASP) questionnaire was distributed to 85 parents of typically developing children (P-TD), 121 parents from simplex ASD families (SPX), and 54 parents from multiplex ASD families (MPX). After controlling for gender and presence of mental disorders, results showed that MPX parents significantly differed from P-TD parents in all four subscales of the AASP. Differences between SPX and MPX parents reached significance in the Sensory Sensitivity subscale and also in subsequent modality-specific analyses in the auditory and visual domains. Our finding that parents with high genetic liability for ASD (i.e., MPX) had more sensory processing atypicalities than parents with low (i.e., SPX) or no (i.e., P-TD) ASD genetic liability suggests that sensory processing atypicalities may contribute to the genetic susceptibility for ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is primarily characterized by social/communication deficits and restricted repetitive behaviors (American Psychiatric Association [APA] 2013). Beginning with the first observations of autism (Asperger 1944; Kanner 1943), it has become well known that many individuals with ASD also have abnormal reactions to sensory input, which include hyperresponsiveness, hyporesponsiveness, and sensory seeking behaviors (e.g., Baranek et al. 2006; Tomchek and Dunn 2007). Evidence of sensory processing abnormalities in individuals with ASD has been demonstrated throughout a variety of measurements and samples consisting of children (Adamson et al. 2006; Baranek et al. 2006; Kirby et al. 2015; Leekam et al. 2007; Tomchek and Dunn 2007; Tomchek et al. 2014) and adults (Cascio et al. 2008; Crane et al. 2009; Grandin 1992; Leekam et al. 2007; Tavassoli et al. 2014) with ASD, including physiological evidence showing hyperresponsive brain activity in reaction to sensory stimuli in ASD youth (Green et al. 2013). Consistent with these findings, “hyper- or hypo-reactivity to sensory input or unusual interests in sensory aspects of the environment” was added as one of the four symptom subcategories defining “repeated, repetitive behaviors” of ASD in the newest version of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5; APA 2013), thus proposing abnormal sensory processing as a significant feature of ASD.
Genetic Influences of Abnormal Sensory Processing and ASD
Considering that ASD has moderate to strong genetic influences (Hallmayer et al. 2011; Ritvo et al. 1985), and that sensory processing abnormalities are shown to be moderately heritable (Goldsmith et al. 2006) and highly prevalent in individuals with ASD (Baranek et al. 2006), it has been suggested that sensory processing abnormalities may share genetic influences with the defining characteristics of ASD (DeLorey et al. 2011; Peñagarikano et al. 2011; Tavassoli et al. 2012). Rodent models of ASD have found that several genetic and epigenetic insults known to produce ASD-like symptoms of social impairments and repetitive/stereotypic behaviors in rodents have also resulted in sensory processing abnormalities (for a review, see Argyropoulos et al. 2013). For instance, Peñagarikano et al. (2011) found that a knockout of CNTNAP2, a well-known ASD candidate gene, resulted in increased thermal and olfactory hypersensitivity in the affected mice. Similarly, DeLorey et al. (2011) found that heterozygosity for the ASD candidate gene GABRB3 in rodents was significantly associated with increased thermal and tactile hypersensitivity. Following these studies, Tavassoli et al. (2012) investigated whether hypersensitivity was also related to GABRB3 variations in humans. They found that behavioral and parent-report measurements of tactile hyperresponsivity in typically developing children were associated with common variations in the GABRB3 candidate gene, thus further supporting genetic implications of sensory processing abnormalities in ASD.
In addition to molecular genetics methods, an alternative method of examining whether particular symptoms are related to genetic influences of ASD is through the assessment of these symptoms in parents and relatives of ASD probands. Relatives of individuals with ASD have an increased chance of displaying mild autistic traits (Bailey et al. 1998; Piven et al. 1997; Szatmari et al. 2008; Taylor et al. 2013), described as the “broader autism phenotype” (BAP; Bolton et al. 1994). BAP traits may represent mild phenotypic expressions of the same genetic influences responsible for ASD (Bailey et al. 1998; Piven 2001). Thus, investigating ASD-like traits in relatives can aid in the search for intermediate phenotypes that may contribute to the genetic liability for ASD (Piven 2001).
The likelihood of BAP traits in relatives is higher in families in which multiple members are diagnosed with ASD (multiple-incidence/multiplex families; MPX) compared to families in which only one relative has ASD (single-incidence/simplex families; SPX), which is likely due to differing genetic mechanisms in these families. While some cases of ASD are heavily influenced by spontaneous de novo mutations (rare genetic mutations present in the child but absent in the parents) of large effect producing sporadic cases of ASD (i.e., SPX) (O’Roak et al. 2012; Sebat et al. 2007), others might be due to the inheritance of ASD-related genes producing familial cases of ASD (i.e., MPX) (Virkud et al. 2009). Supporting the former hypothesis, Sebat et al. (2007) found that the rate of de novo copy number variants was significantly higher in ASD probands from SPX (10 %) compared to those from MPX (3 %) families and control families with no diagnoses of autism (1 %).
Additional evidence supporting the genetic distinction between MPX and SPX families derives from several studies showing that ASD-related traits were more common in relatives from MPX families than those from SPX families (e.g., Bernier et al. 2012; Losh et al. 2008), implying that ASD in the former group is strongly influenced by inheritance of familial ASD traits. Szatmari et al. (2000) evaluated ASD traits in 1362 biological relatives of 78 ASD probands and found that social impairments were significantly more prominent in MPX than in SPX relatives (p < .001), and MPX relatives were also more likely than SPX to have impairments in two or more areas of the three primary symptom categories of ASD (p = .05). Subsequent studies found similar results, showing that, compared to SPX relatives, MPX relatives (usually parents and/or unaffected siblings) had worse pragmatic language and lower quality friendships (Losh et al. 2008), less social interest and less non-verbal communication expression (Gerdts et al. 2013), less social motivation (Bernier et al. 2012), and scored higher on the Social Responsiveness Scale (SRS), signifying more ASD-related deficits (Constantino et al. 2010). A recent study by Oerlemans et al. (2015) also found significant differences in the number of autistic traits between SPX and MPX unaffected siblings, but not between unaffected SPX/MPX parents. Only one study failed to find differences in autistic traits between MPX and SPX families, although they did find marginally significant differences between MPX fathers and control fathers on the SRS (De la Marche et al. 2011). To date, no study has evaluated sensory processing atypicalities in MPX versus SPX families, and therefore it is currently unclear if this common ASD symptom may contribute to the genetic liability for familial cases of ASD.
Abnormal Sensory Processing in Relatives of Individuals with ASD
Two recent studies have evaluated sensory processing in relatives of ASD individuals (De la Marche et al. 2012; Uljarević et al. 2014), although neither used the MPX/SPX distinction outlined above. Both studies used the adolescent/adult sensory profile (AASP) self-report questionnaire, which consists of four quadrants (subscales) corresponding to different types of sensory reactions: hyposensitivity, hypersensitivity, sensation seeking and sensation avoidance (Brown and Dunn 2002). De la Marche et al. (2012) compared 56 non-affected adolescent siblings of ASD individuals with 33 adolescent control participants, and found similar AASP scores between groups except for the Sensation Seeking quadrant, in which siblings of ASD individuals scored significantly lower than adolescent controls (p < .001). The authors concluded that decreased sensation seeking behaviors might be an endophenotypic trait of ASD.
Only one study to our knowledge has investigated sensory processing atypicalities in parents of children with ASD. Uljarević et al. (2014) found that 49 of the 50 mothers (98 %) of children with ASD scored one or more standard deviations (SD) outside the AASP normative means on at least one quadrant of the AASP, and 22 (44 %) scored two or more SDs outside the norms. While Uljarević et al.’s study produced valuable initial discoveries, they only included descriptive findings that compared their sample to the normative means and abnormal classifications found in the AASP manual. As such, a carefully controlled comparison group is needed in order to clearly understand the extent to which sensory processing in parents of ASD children differs from parents of typically developing (TD) children.
In addition, Uljarević et al. (2014) did not control for factors previously associated with scores on self-report sensory processing questionnaires, such as gender and presence of mental disorders, and thus it is unclear if these factors could partially explain their results. Females are known to have a higher sensitivity than males in several modalities (for a review, see Velle 1987). Three recent studies using self-report questionnaires have further supported gender differences in sensory processing, finding that females reported significantly more sensory processing atypicalities than males (Engel-Yeger 2012; Horder et al. 2014; Tavassoli et al. 2014).
Sensory processing abnormalities have been associated with various mental disordersFootnote 1 and disorder symptoms, including ADHD (Mangeot et al. 2001; Ashburner et al. 2008), bipolar disorder (Brown et al. 2002), obsessive–compulsive disorder (OCD; Dar et al. 2012; Rieke and Anderson 2009), post-traumatic stress disorder (PTSD; Engel-Yeger et al. 2013), schizophrenia (Brown et al. 2002), anxiety (Engel-Yeger and Dunn 2011; Horder et al. 2014; Liss et al. 2005) and depression (Liss et al. 2005). In addition, relatives of ASD probands are known to have increased rates of several mental disorders that are associated with sensory symptoms, including OCD (Bolton et al. 1998), affective disorders (Bolton et al. 1998; Piven and Palmer 1999), bipolar disorders (DeLong and Nohna 1994), and schizophrenia (Daniels et al. 2008). Therefore, in order to disentangle influences specific to ASD, it is crucial to control for presence of mental disorders when investigating sensory processing in relatives of individuals with ASD.
Purpose of the Study
The purpose of the present study was to examine how sensory processing differs in parents of children with ASD from SPX and MPX families compared to parents of TD children, in order to gain further insight into the heritable links between sensory symptoms and ASD. This study sought to expand Uljarević et al.’s (2014) initial findings by comparing parents of ASD children with a control group, including fathers in both groups, and examining confounding variables such as gender and presence of mental disorders. In addition, this study aimed to further differentiate the effects of genetic liability for ASD by separating the parents of ASD children into pre-defined SPX and MPX parent groups.
In the present study, the AASP was used to measure sensory processing in parents to allow for direct comparison of this study’s results with the two past studies exploring atypical sensory processing in relatives of individuals with ASD (De la Marche et al. 2012; Uljarević et al. 2014). Based on past research demonstrating that sensory processing abnormalities are heritable (Goldsmith et al. 2006) and related to ASD candidate genes (DeLorey et al. 2011; Peñagarikano et al. 2011; Tavassoli et al. 2012), it was hypothesized that parents of ASD children who presumably have the highest genetic liability for ASD (MPX) would differ from parents with lower ASD genetic liability (SPX) and parents with no such liability (parents of typically developing children; P-TD) in sensory processing scores on all four quadrants of the AASP.
Methods
Participants
Three groups of participants were recruited: parents from multiplex ASD families, parents from simplex ASD families, and parents who had a TD child with no biological ASD relatives. Participants were excluded from the study if they had their own diagnosis of ASD (n = 8), and one participant was excluded due to extreme outlier scores on two of the outcome measures (through use of the outlier labeling rule of Hoaglin and Iglewicz 1987), which suggested rushing or response bias.
Participants were included in the P-TD group if they were the biological parent of at least one typically developing child 4+ years old. Participants were excluded from the P-TD group if any of their biological children had a developmental disorder (n = 16) or if there were suspicions that their child might have ASD (n = 12). As this study focused on traits in ASD-affected families compared to TD families, participants in the P-TD group who had a biologically-related first-, second-, or third-degree family member with ASD were also excluded (n = 7), thus leaving 85 participants in the P-TD group.
Participants were included in the ASD-affected parent groups (MPX or SPX) if they reported they were the biological parent of at least one child 4+ years old who was diagnosed with ASD by a licensed professional (or a previous DSM classification name, such as Asperger’s syndrome, autism disorder, or pervasive developmental disorder-not otherwise specified). In order to include a wide range of severity, it was not made prerequisite that the ASD child was free of comorbid disorders or intellectual disability.
Participants from the ASD-affected group were designated to the SPX group if they (1) had only one biological child with ASD, (2) had at least one biological child without ASD, and (3) had no biological first-, second- or third-degree relatives with ASD. Therefore, singleton ASD families were excluded from this group (n = 48), which left 121 parents in the SPX group. While previous ASD studies included only families with two or more biological ASD siblings in their MPX group (e.g., Losh et al. 2008), we used a more liberal definition of MPX and, in addition to previous criteria, we also included parents who had one biological child with ASD and additionally had one or more first-, second-, or third-degree family members diagnosed with ASD who was biologically related to both the participant and to his or her child with ASD. Although the present study is the first to our knowledge to apply this expanded MPX definition to ASD families, various studies in other fields have used a similar definition of MPX, in which MPX families consisted of two or more first- or second-degree related family members (e.g., Blouin et al. 1998; Michel et al. 2001). As the purpose of the SPX/MPX separation is to represent the underlying genetic distinction between sporadic and familial cases of ASD, we chose this broader definition of MPX because the chances are extremely low that two or more cases of ASD in one biological family are both due to independently occurring rare de novo mutations that similarly resulted in ASD expression. Therefore, by broadening the definition to include any biological relative with ASD, not just a sibling, we maximize the chances of including all familial cases of ASD on the presumption that two cases in one biological family are most likely due to commonly inherited ASD traits. Nevertheless, in order to ensure that this method of distinction was valid, preliminary analyses were run to investigate whether those in the traditional definition of MPX (two or more ASD siblings in one nuclear family; n = 30) differed from those in the broader definition (one ASD child + one ASD family member biologically related to the ASD child and the parent; n = 24). No significant differences were found between these two groups on any outcome measures (.45 ≤ p ≤ .79), and therefore participants were confidently pooled together to form one MPX parent group (n = 54).
Table 1 shows the demographics of the P-TD (n = 85), SPX (n = 121), and MPX (n = 54) parent groups. Chi square tests of independence for each demographic variable showed that the three groups did not significantly differ in regard to gender, country of residence, education level, or amount of parents in each group currently diagnosed with a mental disorder (all ps > .05). The three groups differed in the amount of parents in the youngest age range (p < .05), with the P-TD group reporting more than expected in this youngest range and the MPX group reporting less than expected. However, as age was found to be unrelated to sensory processing scores in young- and middle-aged adult populations in three past studies all using different sensory questionnaires including the AASP (Crane et al. 2009; Robertson and Simmons 2013; Tavassoli et al. 2013), this significant difference in age between the P-TD and MPX groups was not problematic for the present study.
Participants were recruited through various methods of asking third party administrators to share the study’s website with potential participants. Recruitment for the majority of the two ASD-affected groups consisted of calling and/or emailing the head of 419 ASD centers, societies, parent support groups, and schools across the United States to request advertisement of the study’s website. Fifty-five groups agreed to participate through either: hanging the study’s flyer in their center; posting the study on their website, social media pages, or online discussion groups; or sending a mass email to all families involved with the organization. The study was also posted on several websites geared toward ASD research or increasing ASD awareness.
Recruitment aiming to gain participants in the P-TD group consisted of convenience sampling by the authors and contacting the head of daycares, kindergarten-12th grade schools, and universities in the United States to advertise the study in the same ways described above. Eight organizations agreed to do so, including two universities who sent mass emails to all their employees. In order to increase sample size, the study was also posted to six websites that aim to recruit research participants. All participants were asked to explain how they found the survey. From this data, it was found that participants from the five recruitment methods (social media post by an organization, flyer, post on a participant recruitment website, convenience sampling, or direct email) did not differ in any outcome measures (all ps > .05).
Materials
Adolescent/Adult Sensory Profile (AASP)
The AASP is one of the most widely used self-report questionnaires to evaluate sensory processing in adults (Brown and Dunn 2002). This questionnaire is based on Dunn’s (1997) model of sensory processing, which explains reactions to sensory input through a four-quadrant model. One dimension of this model is dedicated to neurological threshold (high threshold: hyposensitive; low threshold: hypersensitive), and the other to behavioral response/self-regulation (active or passive response to the given threshold). There is one subscale for each of the four quadrants, which consist of low registration (LR; high threshold/passive behavioral response), Sensation Seeking (S. Seeking; high threshold/active behavioral response), Sensory Sensitivity (S. Sensitivity; low threshold/passive behavioral response), and Sensation Avoidance (S. Avoidance; low threshold/active behavioral response).
The AASP has 60 items (15 items for each quadrant subscale), which are dispersed throughout six sensory modalities (Taste/Smell, Auditory, Visual, Tactile, Movement, and Activity). Participants respond on an increasing five-point scale how often they behave in the way described by the item (Almost Never, Seldom, Occasionally, Frequently, or Almost Always). Scores for each quadrant range from 15 to 75. An example item is: “I stay away from noisy settings.”
Standard errors of measurement of the AASP range from 3.58 to 4.51 (Brown and Dunn 2002), and internal consistency was found to be good for LR (α = .78), adequate for S. Seeking (α = .60), and good for S. Sensitivity (α = .78) and S. Avoidance (α = .77) (Brown et al. 2001). In the present sample, internal consistencies of the AASP quadrants were similar to Brown et al.’s (2001) results, with good reliability for LR (α = .78), S. Seeking (α = .70), S. Sensitivity (α = .82), and S. Avoidance (α = .84). Supporting concurrent validity of the AASP, scores in the low threshold quadrants (S. Sensitivity and S. Avoidance) were shown to have strong correlations with another self-report questionnaire of hypersensitivity/over-responsivity in adults, the Sensory Over-Responsivity (SensOR) Inventory: SensOR versus S. Sensitivity: r = .74; SensOR versus S. Avoidance: r = .64; both ps < .001 (Schoen et al. 2008).
Using a sample of 496 participants without disabilities aged 18–64 years old, Brown and Dunn (2002) have produced normative means with five classification groups of scores corresponding to how much an individual score differs from the normative mean for each quadrant. The five classification groups follow a normal distribution for each quadrant, for which a score below the 2nd percentile is considered “Much Less than Most People,” a score between the 2nd and 16th percentile is “Less than Most People,” between the 16th and 84th percentile is “Similar to Most People,” between the 84th and 98th percentile is “More than Most People,” and a score above the 98th percentile is “Much More than Most People.”
Demographics/Background Questionnaire
The demographics questionnaire inquired about general characteristics of the participant, such as gender, age group, and education level, but also characteristics specific to this study, such as number of biological children and family history of ASD. As it was not possible to conduct clinical diagnostic methods due to the online format of this study, participants were asked to personally report if they had ever been diagnosed with any mental disorder, and if so, which disorder(s). They were also asked which disorders, if any, they were currently diagnosed with.
Procedure
This study was part of a larger project consisting of six questionnaires total, one of which was the AASP. All validated questionnaires, including the AASP, were entered in their original versions into the online software program Qualtrics.com (Qualtrics, LLC 2015). The total survey took approximately 35–40 min, while the parts relevant to the present study, the AASP and demographics questionnaire, took approximately 10–12 min. To be included in the present study, only completion of the AASP and demographics questionnaires was required. The survey link was posted on a one-page website, which included a short description of the study, contact information, and inclusion criteria. Participants who completed the questionnaire were entered in a raffle to win one of two $50 gift cards (or the equivalent amount in the participant’s home currency).
This study was approved by the Ethical Committee Psychology at Maastricht University (ECP-147_10_12_2014). Informed consent was obtained from all individual participants included in the study.
Data Analyses
Only participants who completed at least 95 % of the required questionnaires were included in data analyses (n = 260).
Descriptive Findings and Preliminary Analyses
In order to compare our results with past studies and the AASP normative means, we first looked at the amount of participants who scored in the extreme outer percentiles of the AASP normative distribution, which referred to scores that were below the 2nd percentile (AASP classification: “Much Less than Most People”) or above the 98th percentile (AASP classification: “Much More than Most People”) for each quadrant. Comparing percentages of extreme scores to those found in the AASP normative sample is common in most studies using the AASP (e.g., Rieke and Anderson 2009; Uljarević et al. 2014), and is a good complementary method in addition to statistically comparing group means.
In order to examine how gender and current presence of mental disorders might influence sensory scores independently from P-TD/SPX/MPX group effects, preliminary analyses consisting of t tests of independent samples were conducted to compare the AASP quadrant scores between genders and also between those with and without a current mental disorder. For these tests, all participants were pooled together and separated based only on the demographic variable in question.
Primary Analyses
To statistically evaluate if sensory processing differed between SPX and MPX parents of ASD children and parents of TD children, we used a one-way multivariate analysis of covariance (MANCOVA) with diagnostic group (P-TD/SPX/MPX) as fixed factor, gender and presence of mental disorders as covariates, and the four AASP quadrant scores as dependent variables. Univariate analyses of covariance (ANCOVAs) were then performed for each quadrant, followed by post hoc analyses using Bonferroni’s correction method for multiple comparisons to determine group differences. Atypical sensory processing was defined as significantly differing from the “typical” group (i.e., P-TD) in any quadrant score.
Secondary Analyses
To determine if any modality in particular was responsible for the MANCOVA results, additional ANCOVAs were performed for each quadrant within each sensory modality (e.g., S. Seeking in the auditory modality, LR in the visual, etc.), followed by post hoc analyses using Bonferroni’s correction method for multiple comparisons when necessary. Participants were only included in this analysis if they had answered all questions in the specific modality and quadrant under investigation. Significance values were set at p ≤ .05 for all preliminary and main analyses, and all tests were performed using the software program, SPSS (Version 21.0).
Results
Descriptive Findings and Preliminary Analyses
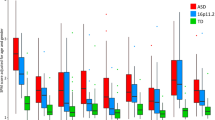
Figure 1 shows the percentage of each group that scored in the extreme outer percentiles in the four quadrants of the AASP. These percentages are compared to those found in the AASP normative sample (i.e., by definition, 4 % of the normative sample scored in the outer two percentiles in each quadrant). In all four quadrants, there was a visible linear trend across families (P-TD < SPX < MPX) in the amount of extreme scores.
Percentage of each group that scored in the extreme outer percentiles of the AASP normative sample scores on four AASP quadrants. Information regarding the AASP norms and the cut-off scores defining the extreme outer percentiles for each quadrant are found in the AASP manual (Brown and Dunn 2002). AASP adolescent/adult sensory profile, P-TD parents of typically developing children, SPX parents of children with autism spectrum disorder (ASD) from simplex families, MPX parents of children with ASD from multiplex families
After assessing the total amount of extreme sensory scores in each group, it was found that 53 % of MPX parents scored in the outer two percentiles in at least one AASP quadrant (28 % had extreme scores in only one quadrant, 15 % in two quadrants, 6 % in three quadrants, and 4 % in all four quadrants). In comparison, 27 % of SPX parents scored in the outer two percentiles in at least one quadrant (10 % in one quadrant, 12 % in two quadrants, and 5 % in three quadrants), and 20 % of P-TD parents scored in this range in at least one quadrant (14 % in one quadrant, 2 % in two quadrants, 4 % in three quadrants).
The preliminary t tests revealed that females scored significantly higher than males in two of the four AASP quadrants: S. Seeking, t(258) = 3.221, p = .001, and S. Sensitivity, t(258) = 3.382, p < .001; while S. Avoidance showed a trend: t(258) = 1.777, p = .07. In addition, parents currently diagnosed with a mental disorder scored significantly higher than parents without such diagnoses in three quadrants: LR, t(258) = 3.688, p < .0001; S. Sensitivity, t(258) = 4.185, p < .0001; and S. Avoidance t(258) = 4.129, p < .0001. Therefore, both gender and presence of a mental disorder were used as covariates in subsequent analyses.
Primary Analyses
All dependent measures for the MANCOVA and the modality-specific ANCOVAs met assumptions for homogeneity (p > .05 for all Levene’s homogeneity of variance tests), thus permitting parametric analyses. Raw scores and standard deviations of each group are presented in Table 2.
The MANCOVA revealed significant differences in AASP scores among the P-TD, SPX, and MPX parent groups, F(8504) = 2.107, p = .034, Wilks’ Lambda = .936; η 2 p = .32. Subsequent ANCOVAs found that scores significantly differed among parent groups in all four sensory quadrants: LR, F(2255) = 3.796, p = .024, η 2 p = .029; S. Seeking, F(2255) = 3.246, p = .041, η 2 p = .025; S. Sensitivity, F(2255) = 5.649, p = .004, η 2 p = .042; and S. Avoidance, F(2255) = 5.825, p = .003, η 2 p = .044. Figure 2 illustrates group differences after correcting for multiple comparisons using Bonferroni’s method.
Pairwise comparisons of AASP quadrant scores among groups. Values shown are estimated marginal means with covariates evaluated at the following: presence of mental disorder = .3423 and gender = 1.14. LR low registration, S sensation/sensory, AASP adolescent/adult sensory profile, P-TD parents of typically developing children, SPX parents of children with autism spectrum disorder (ASD) from simplex families, MPX parents of children with ASD from multiplex families. *p < .05; **p < .01
Secondary Analyses
As shown in Table 3, results of the modality-specific ANCOVAs and Bonferroni corrected post hoc analyses revealed that MPX parents scored significantly higher than both SPX and P-TD parents in the auditory total score, auditory S. Sensitivity, auditory S. Avoidance, visual total score, visual LR, and visual S. Avoidance (all ps < .005), and MPX parents also scored higher than P-TD parents in auditory LR, activity LR, touch S. Sensitivity, and visual S. Sensitivity (all ps < .05). In addition, it was found that SPX parents scored higher than P-TD in auditory LR (p < .01), while P-TD parents scored higher than SPX in the movement total score (p < .05).
Discussion
This study investigated the hypothesis that sensory processing, as measured by the adolescent/adult sensory profile, would differ among parents of typically developing children and parents of children with ASD from SPX and MPX families. Results supported this hypothesis by showing that, after controlling for gender and mental disorders, MPX parents scored significantly lower than P-TD parents in Sensory Seeking, and significantly higher than P-TD parents in the Low Registration, Sensation Avoidance, and Sensory Sensitivity quadrants of the AASP. Upon investigating each modality separately, it was found that the primary results were influenced mostly by scores in the auditory and visual modalities. Differences between SPX and MPX parents reached significance in the Sensory Sensitivity quadrant of the primary analyses, and also in various auditory and visual quadrant scores of the secondary analyses. Our findings that parents with high genetic liability for ASD-related genes (MPX) had more sensory atypicalities than parents with low (SPX) or no (P-TD) genetic liability for ASD suggest that atypical sensory processing may contribute to the genetic susceptibility for ASD. Our conclusions align with recent genetics research suggesting that sensory processing atypicalities may share genetic influences with ASD (DeLorey et al. 2011; Peñagarikano et al. 2011; Tavassoli et al. 2012). Whether sensory processing atypicalities represent an increased risk for ASD specifically, or instead an increased risk for a range of mental disorders (only one of which is ASD), is yet to be verified.
Results from the present study confirm Uljarević et al.’s (2014) past findings that parents of children with ASD had more sensory processing atypicalities than the norm. Although we replicated these general findings, effects in our study were found only in MPX parents of ASD children, while SPX parents scored similarly to P-TD parents. Uljarević et al.’s finding that 44 % of mothers of ASD children scored in the extreme outer percentiles (i.e., > 2 SDs outside the norm) in at least one quadrant is somewhat similar to our results concerning the percentage of MPX parents that scored in the extreme outer percentiles (53 %), but in contrast to results from the SPX (27 %) and P-TD (20 %) parent groups in our study. It is crucial to note that these percentages are not corrected for potentially confounding variables, namely gender and mental disorders, and therefore it is difficult to make valid comparisons based on these data alone. However, after stringent statistical testing including controlling for these variables, we continued to find effects in MPX parents only, whereas scores from SPX parents did not statistically differ from P-TD parents in any primary analysis. Our results showing increased sensory processing atypicalities in MPX parents compared to SPX and P-TD parents offer genetic explanations for Uljarević et al.’s findings, and also support De la Marche et al.’s (2012) conclusion that decreased Sensation Seeking may be an intermediate phenotype of ASD.
Our findings that the auditory and visual modalities showed the largest group effects may be due the crucial involvement of auditory and visual processing in social communication. Successful interpretation of social communication relies on sufficient processing of auditory cues, visual cues, and audio–visual integration, thus emphasizing the relevance of atypical auditory and visual processing in ASD studies. In addition to ASD-specific explanations, it is also possible that group effects were found mostly in the auditory and visual modalities due to factors relating to self-report sensory questionnaires. Considering that reactions experienced in the auditory and visual modalities are verbalized more often than other modalities, participants may have more difficulty in identifying, recalling, or reporting reactions experienced in the other senses.
Our preliminary findings concerning effects of mental disorders are consistent with previous studies that found a relationship between sensory processing abnormalities and various types of mental disorders and disorder symptoms (Ashburner et al. 2008; Brown et al. 2002; Engel-Yeger and Dunn 2011; Engel-Yeger et al. 2013; Mangeot et al. 2001; Rieke and Anderson 2009). Given that (a) ASD probands and their families have an increased chance of having other heritable mental disorders in addition to ASD (e.g., Bolton et al. 1998; Daniels et al. 2008; DeLong and Nohna 1994; Piven and Palmer 1999) and that (b) many of these mental disorders are also related to atypical sensory processing (e.g., Brown et al. 2002; Engel-Yeger et al. 2013; Rieke and Anderson 2009), it remains uncertain whether the increased sensory processing atypicalities observed in MPX parents in our study are related to an increase in genetic susceptibility for ASD specifically, or instead are related to an increased risk for a range of mental disorders. Regardless of whether sensory processing atypicalities are specific to ASD, our findings nevertheless support previous evidence implying that abnormal sensory processing and ASD may share genetic influences.
Preliminary results from this study support recent studies showing that females scored significantly higher than males on sensory processing questionnaires (Engel-Yeger 2012; Horder et al. 2014; Tavassoli et al. 2014), which contributes to the accumulating evidence demonstrating gender differences in sensory processing (Velle 1987). In addition to gender differences that are specific to the female sensory systems, it is also possible that our results concerning gender effects may be partially due to cognitive differences or differences in self-disclosure. For instance, females are found to have a better memory for recognizing and identifying odors (Brand and Millot 2001) and are more likely to disclose personal information than males (Dindia and Allen 1992). Although these explanations might account for the gender effects found in our study and are interesting for future investigations, they cannot explain our main conclusions concerning differences in ASD genetic liability, considering that gender was distributed evenly among all three groups and was controlled for in all main analyses.
Throughout all analyses, there was a linear (though at times non-significant) trend in which atypical sensory scores increased as the amount of presumed genetic liability for ASD increased: P-TD parents had fewer sensory atypicalities than SPX parents, who in turn had less sensory atypicalities than MPX parents. A likely explanation for the slight increase in scores in the SPX group is that some parents who were originally designated to this group might in fact have ASD genetic mechanisms more similar to the traditional assumptions of MPX families (i.e., familial ASD-related traits) than those of SPX (i.e., isolated genetic mutations), which is a common concern in SPX/MPX studies (e.g., Klei et al. 2012). In addition to general uncertainty of mental disorder status of all biological relatives, another factor contributing to group misplacement could be the “stoppage effect,” which occurs when a family ceases child-bearing after an ASD diagnosis is discovered in one of their children (Jones and Szatmari 1988). This effect questions a family’s SPX classification, as it is unknown how future children would have developed. Another possible explanation for group misplacement is that some relatives within SPX families could have pronounced ASD traits without an official ASD diagnosis, and therefore would not be recognized in our family history questionnaire. SPX families with prevalent ASD traits (albeit no official diagnoses) would better fit the genetic profile of MPX families than that of SPX. Considering these various situations, it is possible that some SPX parents may have had unrealized MPX status, thus causing a slight increase in overall sensory atypicalities in this group.
Past studies investigating intermediate phenotypes in relatives of ASD individuals have already identified numerous heritable traits that may increase genetic vulnerability for ASD, including social/emotional abnormalities (e.g., Gerdts et al. 2013), pragmatic language impairments (e.g., Whitehouse et al. 2007), stereotyped behaviors (e.g., Piven et al. 1997), and even certain personality traits (Losh et al. 2008; Murphy et al. 2000; Piven et al. 1994). Findings from the present study suggest that atypical sensory processing might be an additional heritable trait contributing to ASD susceptibility. Assuming that each heritable trait of ASD is expressed as a result of variations in specific genes related to ASD susceptibility (Piven 2001), then it is plausible that an increase in the number of inherited ASD-related genetic variations would additively increase vulnerability of developing ASD. Along these lines, Klei et al. (2012) found that many common genetic variations that have small effect in isolation, have large effects on ASD susceptibility when acting additively. These additive effects were found more often in relatives from MPX ASD families (60 %) than from SPX ASD families (40 %), thus implying that ASD cases from MPX families may result largely from the additive effect of numerous ASD-related genetic variations of small influence. It may be that some of these common genetic variations give rise to the ASD-like traits often observed in relatives of ASD probands. Considering that sensory processing atypicalities are now part of the ASD diagnostic category of restricted repetitive behaviors (APA 2013) and are strongly associated with autistic traits in both ASD (Boyd et al. 2009; Wigham et al. 2015) and non-ASD populations (Robertson and Simmons 2013; Horder et al. 2014), the results from the present study imply that atypical sensory processing might be an additional potential BAP trait worthy of future investigations, which could further aid in the search for genetic variations responsible for ASD.
In addition to theoretical implications concerning atypical sensory processing and ASD genetic liability, the current study also has important practical implications. Regardless of the exact causes behind these results, our findings show that many parents of ASD children perceive the sensory world differently than most people, which, in extreme cases, could result in difficulties in many areas of daily functioning. According to personal accounts from individuals with ASD, severe sensory reactions can encourage social withdrawal and reduce participation in many activities (Grandin 1992; Kirby et al. 2015), a consequence which influences the individual’s general social/communication functioning. These consequences may also apply to some MPX parents, considering that the overall pattern of sensory atypicalities seen in MPX parents in this study (i.e., lower than controls in S. Seeking and higher in the remaining three quadrants) directly corresponds to the sensory pattern found in adults diagnosed with ASD (Crane et al. 2009).
Apart from affecting social functioning, sensory processing difficulties have also been associated with anxiety symptoms (Engel-Yeger and Dunn 2011; Kinnealey and Fuiek 1999), depression symptoms (Liss et al. 2005; Kinnealey and Fuiek 1999), sleep quality (Engel-Yeger and Shochat 2012), and even physical health symptoms (Benham 2006). Although these associations do not imply causation, they nevertheless demonstrate that many parents of ASD children who have sensory symptoms likely have additional problems affecting their well-being. While it is standard practice to treat sensory symptoms in children diagnosed with ASD, the present study suggests that their parents could also benefit from understanding and alleviating their own sensory difficulties. This would not only improve the parent’s well-being, but could also improve their child’s well-being, by enabling a more nurturing and enriching environment in which the child could develop into his or her highest potential.
Although this study’s sample size was relatively large [i.e., three times the size of past studies using the AASP in ASD relatives (De la Marche et al. 2012; Uljarević et al. 2014)], thus emphasizing the validity of these findings, there are a few limitations concerning the sample that should be addressed. First, we relied on self-reports of mental diagnoses of the participant, their children, and—due to our expanded definition of MPX families—their biological relatives. Validity of these diagnoses is crucial, as the diagnostic statuses of the participant’s child and biological relatives created the distinction among the three study groups, and the participant’s own diagnoses were found to have large effects on AASP scores in preliminary analyses. Diagnostic evaluations by clinicians of the parents, their children, and their relatives would have been better to ensure accuracy of ASD diagnoses in the ASD groups, and also to guarantee a well-controlled comparison group. However, we did make an effort to alleviate the latter concern by excluding parents from the P-TD group who had suspicions that their child might have ASD or who reported that they had a biological relative with ASD. In addition, it is important to note that participants in the ASD-affected parent groups were recruited from official ASD organizations where diagnoses by a licensed professional are administered, required, or assumed. Therefore, although ASD diagnoses of the children with ASD could not be directly confirmed, our manner of recruitment increases confidence that a participant’s child likely received a confirmed diagnosis of ASD by a licensed professional if the parent reported so on the questionnaire.
Clinical diagnostic evaluations of the participants would have not only confirmed their current mental disorders, but could have also provided information regarding their sub-clinical disorder symptoms. As sensory processing abnormalities were found to be associated with symptoms of several mental disorders (e.g., Dar et al. 2012; Engel-Yeger and Dunn 2011; Horder et al. 2014; Liss et al. 2005), it remains uncertain whether subclinical symptoms in the parents could have partially explained our results. Addressing this limitation in future research is imperative in order to better understand the extent to which sensory processing abnormalities are specific to ASD genetic liability.
A general limitation of using sensory questionnaires is the reliance on self-reported subjective reactions to sensory experiences, which requires honest and reliable introspection. Furthermore, the AASP has been criticized for including sensory items that are associated with affective or social reactions (Tavassoli et al. 2014). For these reasons, supplementary sensory processing measurements involving objective physiological tests are recommended to accompany self-report questionnaires, although this was unfortunately not an option for the present online study. Despite potential limitations of the AASP, the present study nonetheless produced significant findings, which is a valuable advancement towards understanding how atypical sensory processing may relate to the genetic liability for ASD.
Future research should replicate the present study’s results with use of professional diagnostic evaluations and additional objective sensory measurements, such as measuring behavioral or physiological reactions to sensory stimuli. Given that this study used online questionnaires to understand traits contributing to ASD genetic liability, evident next steps also include genetic association studies to discover the genes underpinning our results, similar to the pilot study by Tavassoli et al. (2012).
Through investigating the many traits of the broader autism phenotype and eventually discovering their genetic etiology, we can improve genetics research and gradually move closer toward a global understanding of ASD. Findings from the present study contribute to this effort by suggesting that atypical sensory processing may be one of the heritable traits contributing to ASD susceptibility, which is worthy of future investigations.
Notes
In this context, a mental disorder refers to any psychological or behavioral syndrome recognized in the DSM (APA 2013) or International Statistical Classification of Diseases and Related Health Problems (World Health Organization 1992) that causes “clinically significant disturbance in an individual’s cognition, emotion regulation, or behavior” (APA 2013).
References
Adamson, A., O’Hare, A., & Graham, C. (2006). Impairments in sensory modulation in children with autistic spectrum disorder. British Journal of Occupational Therapy, 69(8), 357–364.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Argyropoulos, A., Gilby, K. L., & Hill-Yardin, E. L. (2013). Studying autism in rodent models: reconciling endophenotypes with comorbidities. Frontiers in Human Neuroscience, 7, 417.
Ashburner, J., Ziviani, J., & Rodger, S. (2008). Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. American Journal of Occupational Therapy, 62(5), 564–573.
Asperger, H. (1944). Die “autistischen psychopathen” im kindesalter. European Archives of Psychiatry and Clinical Neuroscience, 117(1), 76–136.
Bailey, A., Palferman, S., Heavey, L., & Le Couteur, A. (1998). Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders, 28(5), 369–392.
Baranek, G. T., David, F. J., Poe, M. D., Stone, W. L., & Watson, L. R. (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601.
Benham, G. (2006). The highly sensitive person: Stress and physical symptom reports. Personality and Individual Differences, 40(7), 1433–1440.
Bernier, R., Gerdts, J., Munson, J., Dawson, G., & Estes, A. (2012). Evidence for broader autism phenotype characteristics in parents from multiple-incidence autism families. Autism Research, 5(1), 13–20.
Blouin, J. L., Dombroski, B. A., Nath, S. K., Lasseter, V. K., Wolyniec, P. S., Nestadt, G., et al. (1998). Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nature Genetics, 20(1), 70–73.
Bolton, P., Macdonald, H., Pickles, A., Rios, P. A., Goode, S., Crowson, M., et al. (1994). A case–control family history study of autism. Journal of Child Psychology and Psychiatry, 35(5), 877–900.
Bolton, P. F., Pickles, A., Murphy, M., & Rutter, M. (1998). Autism, affective and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine, 28(2), 385–395.
Boyd, B. A., McBee, M., Holtzclaw, T., Baranek, G. T., & Bodfish, J. W. (2009). Relationships among repetitive behaviors, sensory features, and executive functions in high functioning autism. Research in Autism Spectrum Disorders, 3(4), 959–966.
Brand, G., & Millot, J. L. (2001). Sex differences in human olfaction: Between evidence and enigma. The Quarterly Journal of Experimental Psychology: Section B, 54(3), 259–270.
Brown, C., Cromwell, R. L., Filion, D., Dunn, W., & Tollefson, N. (2002). Sensory processing in schizophrenia: Missing and avoiding information. Schizophrenia Research, 55(1), 187–195.
Brown, C., & Dunn, W. (2002). Adolescent–adult sensory profile: User’s manual. San Antonio, TX: Psychological Corporation.
Brown, C., Tollefson, N., Dunn, W., Cromwell, R., & Filion, D. (2001). The adult sensory profile: Measuring patterns of sensory processing. American Journal of Occupational Therapy, 55(1), 75–82.
Cascio, C., McGlone, F., Folger, S., Tannan, V., Baranek, G., Pelphrey, K. A., et al. (2008). Tactile perception in adults with autism: A multidimensional psychophysical study. Journal of Autism and Developmental Disorders, 38(1), 127–137.
Constantino, J. N., Zhang, Y., Frazier, T., Abbacchi, A. M., & Law, P. (2010). Sibling recurrence and the genetic epidemiology of autism. The American Journal of Psychiatry, 167(11), 1349–1356.
Crane, L., Goddard, L., & Pring, L. (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13(3), 215–228.
Daniels, J. L., Forssen, U., Hultman, C. M., Cnattingius, S., Savitz, D. A., Feychting, M., et al. (2008). Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics, 121(5), 1357–1362.
Dar, R., Kahn, D. T., & Carmeli, R. (2012). The relationship between sensory processing, childhood rituals and obsessive–compulsive symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 43(1), 679–684.
De la Marche, W., Noens, I., Luts, J., Scholte, E., Van Huffel, S., & Steyaert, J. (2011). Quantitative autism traits in first degree relatives: Evidence for the broader autism phenotype in fathers, but not in mothers and siblings. Autism, 16(3), 247–260.
De la Marche, W., Steyaert, J., & Noens, I. (2012). Atypical sensory processing in adolescents with an autism spectrum disorder and their non-affected siblings. Research in Autism Spectrum Disorders, 6(2), 639–645.
DeLong, R., & Nohna, C. (1994). Psychiatric family history and neurological disease in autistic spectrum disorders. Developmental Medicine and Child Neurology, 36(5), 441–448.
DeLorey, T. M., Sahbaie, P., Hashemi, E., Li, W. W., Salehi, A., & Clark, D. J. (2011). Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behavioural Brain Research, 216(1), 36–45.
Dindia, K., & Allen, M. (1992). Sex differences in self-disclosure: A meta-analysis. Psychological Bulletin, 112(1), 106–124.
Dunn, W. (1997). The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants and Young Children, 9(4), 23–35.
Engel-Yeger, B. (2012). Validating the adolescent/adult sensory profile and examining its ability to screen sensory processing difficulties among Israeli people. The British Journal of Occupational Therapy, 75(7), 321–329.
Engel-Yeger, B., & Dunn, W. (2011). The relationship between sensory processing difficulties and anxiety level of healthy adults. The British Journal of Occupational Therapy, 74(5), 210–216.
Engel-Yeger, B., Palgy-Levin, D., & Lev-Wiesel, R. (2013). The sensory profile of people with post-traumatic stress symptoms. Occupational Therapy in Mental Health, 29(3), 266–278.
Engel-Yeger, B., & Shochat, T. (2012). The relationship between sensory processing patterns and sleep quality in healthy adults. Canadian Journal of Occupational Therapy, 79(3), 134–141.
Gerdts, J. A., Bernier, R., Dawson, G., & Estes, A. (2013). The broader autism phenotype in simplex and multiplex families. Journal of Autism and Developmental Disorders, 43(7), 1597–1605.
Goldsmith, H. H., Van Hulle, C. A., Arneson, C. L., Schreiber, J. E., & Gernsbacher, M. A. (2006). A population-based twin study of parentally reported tactile and auditory defensiveness in young children. Journal of Abnormal Child Psychology, 34(3), 378–392.
Grandin, T. (1992). An inside view of autism. In E. Schopler & G. B. Mesibov (Eds.), High-functioning individuals with autism (pp. 105–126). New York, NY: Plenum Press.
Green, S. A., Rudie, J. D., Colich, N. L., Wood, J. J., Shirinyan, D., Hernandez, L., et al. (2013). Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52(11), 1158–1172.
Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., & Risch, N. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095–1102.
Hoaglin, D. C., & Iglewicz, B. (1987). Fine tuning some resistant rules for outlier labeling. Journal of American Statistical Association, 82(400), 1147–1149.
Horder, J., Wilson, C. E., Mendez, M. A., & Murphy, D. G. (2014). Autistic traits and abnormal sensory experiences in adults. Journal of Autism and Developmental Disorders, 44(6), 1461–1469.
Jones, M. B., & Szatmari, P. (1988). Stoppage rules and genetic studies of autism. Journal of Autism and Developmental Disorders, 18(1), 31–40.
Kanner, L. (1943). Autistic disturbances of affective contact. Nervous Child: Journal of Psychopathology, Psychotherapy, Mental Hygiene, and Guidance of the Child, 2, 217–250.
Kinnealey, M., & Fuiek, M. (1999). The relationship between sensory defensiveness, anxiety, depression and perception of pain in adults. Occupational Therapy International, 6(3), 195–206.
Kirby, A. V., Dickie, V. A., & Baranek, G. T. (2015). Sensory experiences of children with autism spectrum disorder: In their own words. Autism, 19(3), 316–326.
Klei, L., Sanders, S. J., Murtha, M. T., Hus, V., Lowe, J. K., Willsey, A. J., et al. (2012). Common genetic variants, acting additively, are a major source of risk for autism. Molecular Autism, 3, 9.
Leekam, S. R., Nieto, C., Libby, S. J., Wing, L., & Gould, J. (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37(5), 894–910.
Liss, M., Timmel, L., Baxley, K., & Killingsworth, P. (2005). Sensory processing sensitivity and its relation to parental bonding, anxiety, and depression. Personality and Individual Differences, 39(8), 1429–1439.
Losh, M., Childress, D., Lam, K., & Piven, J. (2008). Defining key features of the broad autism phenotype: A comparison across parents of multiple-and single-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 147(4), 424–433.
Mangeot, S. D., Miller, L. J., McIntosh, D. N., McGrath-Clarke, J., Simon, J., Hagerman, R. J., et al. (2001). Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology, 43(6), 399–406.
Michel, M., Johanet, C., Meyer, O., Frances, C., Wittke, F., Michel, C., et al. (2001). Familial lupus erythematosus: Clinical and immunologic features of 125 multiplex families. Medicine, 80(3), 153–158.
Murphy, M., Bolton, P. F., Pickles, A., Fombonne, E., Piven, J., & Rutter, M. (2000). Personality traits of the relatives of autistic probands. Psychological Medicine, 30(6), 1411–1424.
O’Roak, B. J., Vives, L., Girirajan, S., Karakoc, E., Krumm, N., Coe, B. P., et al. (2012). Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature, 485(7397), 246–250.
Oerlemans, A. M., Hartman, C. A., De Bruijn, Y. G., Van Steijn, D. J., Franke, B., Buitelaar, J. K., et al. (2015). Simplex and multiplex stratification in ASD and ADHD families: A promising approach for identifying overlapping and unique underpinnings of ASD and ADHD? Journal of Autism and Developmental Disorders, 45(3), 645–657.
Peñagarikano, O., Abrahams, B. S., Herman, E. I., Winden, K. D., Gdalyahu, A., Dong, H., et al. (2011). Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell, 147(1), 235–246.
Piven, J. (2001). The broad autism phenotype: a complementary strategy for molecular genetic studies of autism. American Journal of Medical Genetics, 105(1), 34–35.
Piven, J., & Palmer, P. (1999). Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry, 156(4), 557–563.
Piven, J., Palmer, P., Jacobi, D., Childress, D., & Arndt, S. (1997). Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry, 154(2), 185–190.
Piven, J., Wzorek, M., Landa, R., Lainhart, J., Bolton, P., Chase, G. A., et al. (1994). Personality characteristics of the parents of autistic individuals. Psychological Medicine, 24(3), 783–795.
Qualtrics, LLC. (2015). Retrieved from http://www.qualtrics.com.
Rieke, E. F., & Anderson, D. (2009). Adolescent/adult sensory profile and obsessive–compulsive disorder. American Journal of Occupational Therapy, 63(2), 138–145.
Ritvo, E. R., Freeman, B. J., Mason-Brothers, A., Mo, A., & Ritvo, A. M. (1985). Concordance for the syndrome of autism in 40 pairs of afflicted twins. American Journal of Psychiatry, 142(1), 74–77.
Robertson, A. E., & Simmons, D. R. (2013). The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental Disorders, 43(4), 775–784.
Schoen, S. A., Miller, L. J., & Green, K. E. (2008). Pilot study of the sensory over-responsivity scales: Assessment and inventory. American Journal of Occupational Therapy, 62(4), 393–406.
Sebat, J., Lakshmi, B., Malhotra, D., Troge, J., Lese-Martin, C., Walsh, T., et al. (2007). Strong association of de novo copy number mutations with autism. Science, 316(5823), 445–449.
Szatmari, P., Georgiades, S., Duku, E., Zwaigenbaum, L., Goldberg, J., & Bennett, T. (2008). Alexithymia in parents of children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 38(10), 1859–1865.
Szatmari, P., MacLean, J. E., Jones, M. B., Bryson, S. E., Zwaigenbaum, L., Bartolucci, G., et al. (2000). The familial aggregation of the lesser variant in biological and nonbiological relatives of PDD probands: A family history study. Journal of Child Psychology and Psychiatry, 41(5), 579–586.
Tavassoli, T., Auyeung, B., Murphy, L. C., Baron-Cohen, S., & Chakrabarti, B. (2012). Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Molecular Autism, 3, 6.
Tavassoli, T., Hoekstra, R. A., & Baron-Cohen, S. (2014). The Sensory Perception Quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Molecular Autism, 5, 29.
Tavassoli, T., Miller, L. J., Schoen, S. A., Nielsen, D. M., & Baron-Cohen, S. (2013). Sensory over-responsivity in adults with autism spectrum conditions. Autism, 18, 428–432.
Taylor, L. J., Maybery, M. T., Wray, J., Ravine, D., Hunt, A., & Whitehouse, A. J. (2013). Brief report: Do the nature of communication impairments in autism spectrum disorders relate to the broader autism phenotype in parents? Journal of Autism and Developmental Disorders, 43(12), 2984–2989.
Tomchek, S. D., & Dunn, W. (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200.
Tomchek, S. D., Huebner, R. A., & Dunn, W. (2014). Patterns of sensory processing in children with an autism spectrum disorder. Research in Autism Spectrum Disorders, 8(9), 1214–1224.
Uljarević, M., Prior, M. R., & Leekam, S. R. (2014). First evidence of sensory atypicality in mothers of children with autism spectrum disorder (ASD). Molecular Autism, 5, 26.
Velle, W. (1987). Sex differences in sensory functions. Perspectives in Biology and Medicine, 30(4), 490–522.
Virkud, Y. V., Todd, R. D., Abbacchi, A. M., Zhang, Y., & Constantino, J. N. (2009). Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150(3), 328–334.
Whitehouse, A. J., Barry, J. G., & Bishop, D. V. (2007). The broader language phenotype of autism: A comparison with specific language impairment. Journal of Child Psychology and Psychiatry, 48(8), 822–830.
Wigham, S., Rodgers, J., South, M., McConachie, H., & Freeston, M. (2015). The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 943–952.
World Health Organization. (1992). The ICD-10 classification of mental and behavioral disorders: Clinical descriptions and Guidelines. Geneva: World Health Organization.
Acknowledgments
This research was supported by a Fulbright Scholarship to CD. The authors would like to thank all the organizations who helped with recruitment of participants, with special thanks to: Dr. Carolyn Garver from the Autism Treatment Center of Dallas; Maryellen Quinn-Lunny, Ed. S., from the Florida Atlantic University Center for Autism and Related Disabilities; and Paul M. Budd, LBSW, from the Texas Department of State Health Services. Many thanks to all the parents in this study for their participation.
Author’s Contribution
CD conceived of and designed the study, performed the measurements and statistical analyses, interpreted the data, and drafted the manuscript. JS participated in the design and coordination of the study, interpretation of the data, and helped revise the manuscript. FD participated in the acquisition and interpretation of the data, and helped revise the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Donaldson, C.K., Stauder, J.E.A. & Donkers, F.C.L. Increased Sensory Processing Atypicalities in Parents of Multiplex ASD Families Versus Typically Developing and Simplex ASD Families. J Autism Dev Disord 47, 535–548 (2017). https://doi.org/10.1007/s10803-016-2888-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-016-2888-0